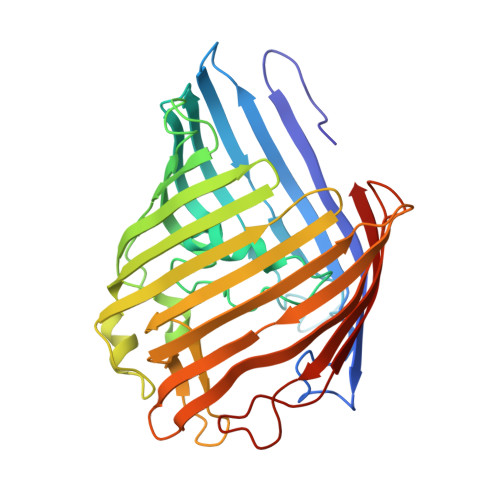
Structures of the OmpF porin crystallized in the presence of foscholine-12.
Kefala, G., Ahn, C., Krupa, M., Esquivies, L., Maslennikov, I., Kwiatkowski, W., Choe, S.(2010) Protein Sci 19: 1117-1125
- PubMed: 20196071
- DOI: https://doi.org/10.1002/pro.369
- Primary Citation of Related Structures:
3K19, 3K1B - PubMed Abstract:
The endogenous Escherichia coli porin OmpF was crystallized as an accidental by-product of our efforts to express, purify, and crystallize the E. coli integral membrane protein KdpD in the presence of foscholine-12 (FC12). FC12 is widely used in membrane protein studies, but no crystal structure of a protein that was both purified and crystallized with this detergent has been reported in the Protein Data Bank. Crystallization screening for KdpD yielded two different crystals of contaminating protein OmpF. Here, we report two OmpF structures, the first membrane protein crystal structures for which extraction, purification, and crystallization were done exclusively with FC12. The first structure was refined in space group P21 with cell parameters a = 136.7 A, b = 210.5 A, c = 137 A, and beta = 100.5 degrees , and the resolution of 3.8 A. The second structure was solved at the resolution of 4.4 A and was refined in the P321 space group, with unit cell parameters a = 215.5 A, b = 215.5 A, c = 137.5 A, and gamma = 120 degrees . Both crystal forms show novel crystal packing, in which the building block is a tetrahedral arrangement of four trimers. Additionally, we discuss the use of FC12 for membrane protein crystallization and structure determination, as well as the problem of the OmpF contamination for membrane proteins overexpressed in E. coli.
- Structural Biology Laboratory, The Salk Institute for Biological Studies, 10010 North Torrey Pines Rd., La Jolla, California 92037, USA.
Organizational Affiliation: