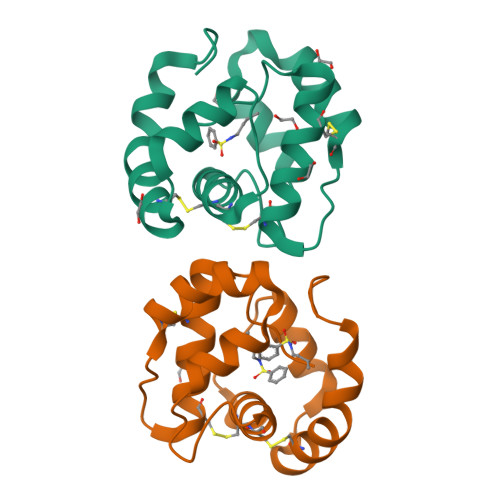
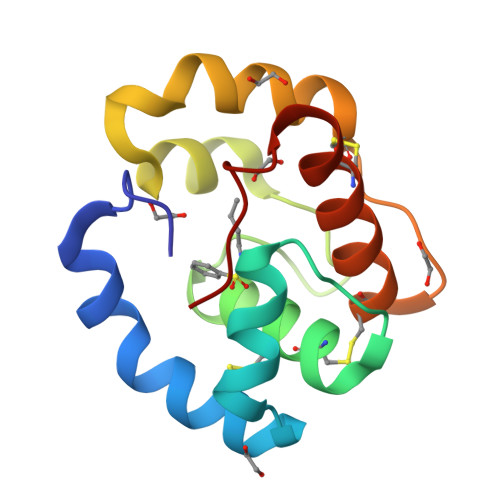
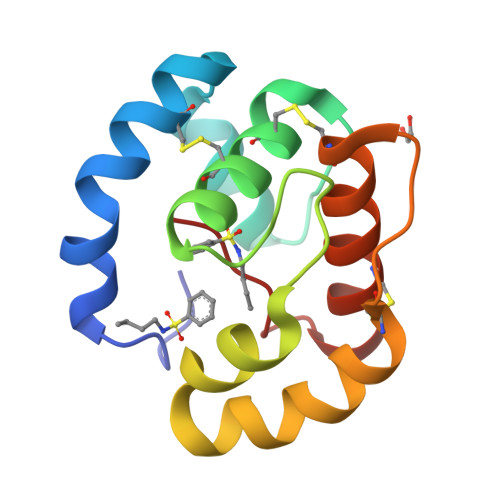
Queen bee pheromone binding protein pH-induced domain swapping favors pheromone release
Pesenti, M.E., Spinelli, S., Bezirard, V., Briand, L., Pernollet, J.C., Campanacci, V., Tegoni, M., Cambillau, C.(2009) J Mol Biology 390: 981-990
- PubMed: 19481550
- DOI: https://doi.org/10.1016/j.jmb.2009.05.067
- Primary Citation of Related Structures:
3CYZ, 3CZ0, 3CZ1, 3CZ2, 3D73, 3D74, 3D75, 3D76, 3D77, 3D78 - PubMed Abstract:
In honeybee (Apis mellifera) societies, the queen controls the development and the caste status of the members of the hive. Queen bees secrete pheromonal blends comprising 10 or more major and minor components, mainly hydrophobic. The major component, 9-keto-2(E)-decenoic acid (9-ODA), acts on the workers and male bees (drones), eliciting social or sexual responses. 9-ODA is captured in the antennal lymph and transported to the pheromone receptor(s) in the sensory neuron membranes by pheromone binding proteins (PBPs). A key issue is to understand how the pheromone, once tightly bound to its PBP, is released to activate the receptor. We report here on the structure at physiological pH of the main antennal PBP, ASP1, identified in workers and male honeybees, in its apo or complexed form, particularly with the main component of the queen mandibular pheromonal mixture (9-ODA). Contrary to the ASP1 structure at low pH, the ASP1 structure at pH 7.0 is a domain-swapped dimer with one or two ligands per monomer. This dimerization is disrupted by a unique residue mutation since Asp35 Asn and Asp35 Ala mutants remain monomeric at pH 7.0, as does native ASP1 at pH 4.0. Asp35 is conserved in only approximately 30% of medium-chain PBPs and is replaced by other residues, such as Asn, Ala and Ser, among others, thus excluding that they may perform domain swapping. Therefore, these different medium-chain PBPs, as well as PBPs from moths, very likely exhibit different mechanisms of ligand release or receptor recognition.
Organizational Affiliation:
Architecture et Fonction des Macromolécules Biologiques, UMR CNRS, Marseille, France.