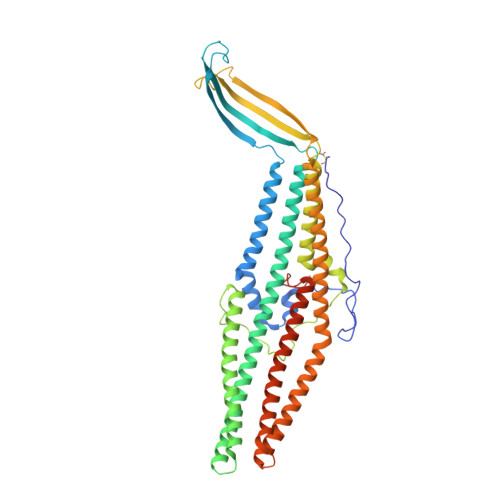
Structural and dynamical insights into the opening mechanism of P. aeruginosa OprM channel.
Phan, G., Benabdelhak, H., Lascombe, M.B., Benas, P., Rety, S., Picard, M., Ducruix, A., Etchebest, C., Broutin, I.(2010) Structure 18: 507-517
- PubMed: 20399187
- DOI: https://doi.org/10.1016/j.str.2010.01.018
- Primary Citation of Related Structures:
3D5K - PubMed Abstract:
Originally described in bacteria, drug transporters are now recognized as major determinants in antibiotics resistance. For Gram-negative bacteria, the reversible assembly consisting of an inner membrane protein responsible for the active transport, a periplasmic protein, and an exit outer membrane channel achieves transport. The opening of the outer membrane protein OprM from Pseudomonas aeruginosa was modeled through normal mode analysis starting from a new X-ray structure solved at 2.4 A resolution in P2(1)2(1)2(1) space group. The three monomers are not linked by internal crystallographic symmetries highlighting the possible functional differences. This structure is closed at both ends, but modeling allowed for an opening that is not reduced to the classically proposed "iris-like mechanism."
- Laboratoire de Cristallographie et RMN Biologiques, Université Paris Descartes, UMR 8015 CNRS, Faculté des Sciences Pharmaceutiques et Biologiques, 4 Avenue de l'Observatoire, 75270 Paris Cedex 06, France.
Organizational Affiliation: