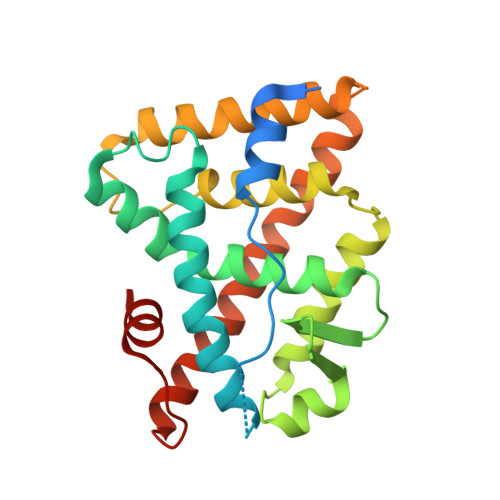
Communication between the ERR{alpha} Homodimer Interface and the PGC-1{alpha} Binding Surface via the Helix 8-9 Loop.
Greschik, H., Althage, M., Flaig, R., Sato, Y., Chavant, V., Peluso-Iltis, C., Choulier, L., Cronet, P., Rochel, N., Schule, R., Stromstedt, P.E., Moras, D.(2008) J Biological Chem 283: 20220-20230
- PubMed: 18441008
- DOI: https://doi.org/10.1074/jbc.M801920200
- Primary Citation of Related Structures:
3D24 - PubMed Abstract:
Although structural studies on the ligand-binding domain (LBD) have established the general mode of nuclear receptor (NR)/coactivator interaction, determinants of binding specificity are only partially understood. The LBD of estrogen receptor-alpha (ERalpha), for example, interacts only with a region of peroxisome proliferator-activated receptor coactivator (PGC)-1alpha, which contains the canonical LXXLL motif (NR box2), whereas the LBD of estrogen-related receptor-alpha (ERRalpha) also binds efficiently an untypical, LXXYL-containing region (NR box3) of PGC-1alpha. Surprisingly, in a previous structural study, the ERalpha LBD has been observed to bind NR box3 of transcriptional intermediary factor (TIF)-2 untypically via LXXYL, whereas the ERRalpha LBD binds this region of TIF-2 only poorly. Here we present a new crystal structure of the ERRalpha LBD in complex with a PGC-1alpha box3 peptide. In this structure, residues N-terminal of the PGC-1alpha LXXYL motif formed contacts with helix 4, the loop connecting helices 8 and 9, and with the C terminus of the ERRalpha LBD. Interaction studies using wild-type and mutant PGC-1alpha and ERRalpha showed that these contacts are functionally relevant and are required for efficient ERRalpha/PGC-1alpha interaction. Furthermore, a structure comparison between ERRalpha and ERalpha and mutation analyses provided evidence that the helix 8-9 loop, which differs significantly in both nuclear receptors, is a major determinant of coactivator binding specificity. Finally, our results revealed that in ERRalpha the helix 8-9 loop allosterically links the LBD homodimer interface with the coactivator cleft, thus providing a plausible explanation for distinct PGC-1alpha binding to ERRalpha monomers and homodimers.
- Département de Biologie et Génomique Structurales, Institut de Génétique et de Biologie Moléculaire et Cellulaire, Illkirch, France.
Organizational Affiliation: