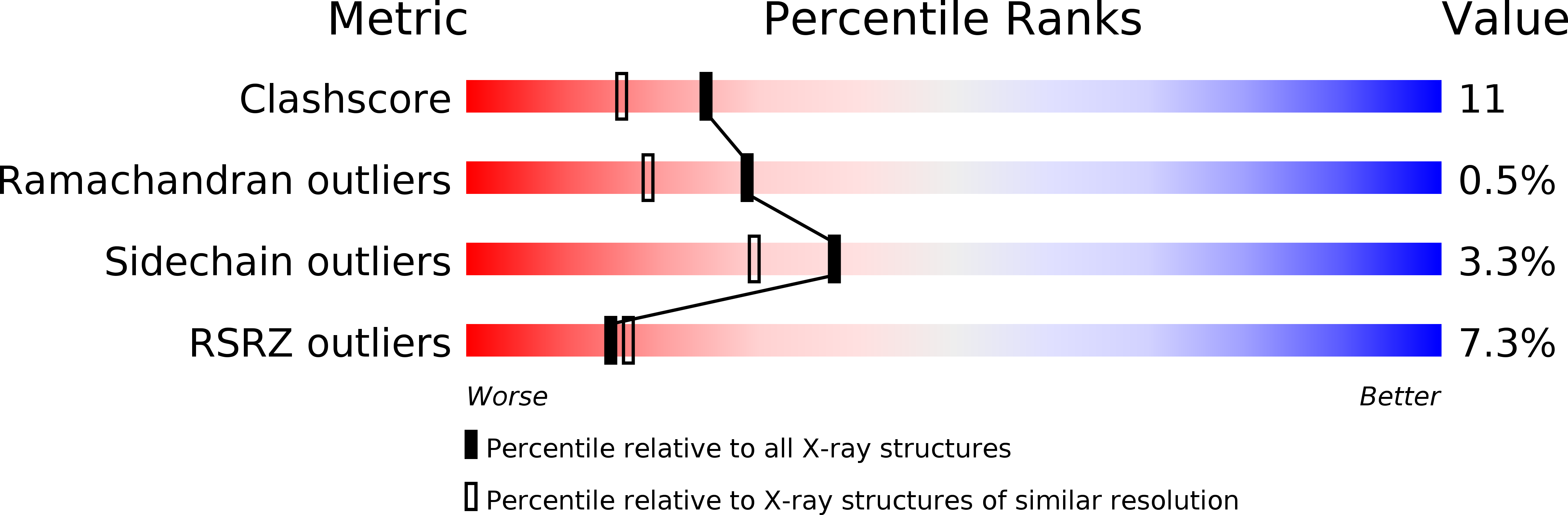
Crystal structures and mutagenesis of sucrose hydrolase from Xanthomonas axonopodis pv. glycines: insight into the exclusively hydrolytic amylosucrase fold.
Kim, M.I., Kim, H.S., Jung, J., Rhee, S.(2008) J Mol Biology 380: 636-647
- PubMed: 18565544
- DOI: https://doi.org/10.1016/j.jmb.2008.05.046
- Primary Citation of Related Structures:
3CZE, 3CZG, 3CZK, 3CZL - PubMed Abstract:
Neisseria polysaccharea amylosucrase (NpAS), a transglucosidase of glycoside hydrolase family 13, is a hydrolase and glucosyltransferase that catalyzes the synthesis of amylose-like polymer from a sucrose substrate. Recently, an NpAS homolog from Xanthomonas axonopodis pv. glycines was identified as a member of the newly defined carbohydrate utilization locus that regulates the utilization of plant sucrose in phytopathogenic bacteria. Interestingly, this enzyme is exclusively a hydrolase and not a glucosyltransferase; it is thus known as sucrose hydrolase (SUH). Here, we elucidated the novel functional features of SUH using X-ray crystallography and site-directed mutagenesis. Four different crystal structures of SUH, including the SUH-Tris and the SUH-sucrose and SUH-glucose complexes, represent structural snapshots along the catalytic reaction coordinate. These structures show that SUH is distinctly different from NpAS in that ligand-induced conformational changes in SUH cause the formation of a pocket-shaped active site and in that SUH lacks the three arginine residues found in the NpAS active site that appear to be crucial for NpAS glucosyltransferase activity. Mutation of SUH to insert these arginines failed to confer glucosyltransferase activity, providing evidence that its enzymatic activity is limited to sucrose hydrolysis by its pocket-shaped active site and the identity of residues in the vicinity of the active site.
Organizational Affiliation:
Department of Agricultural Biotechnology and Center for Agricultural Biomaterials, College of Agriculture and Life Sciences, Seoul National University, Seoul 151-921, Korea.