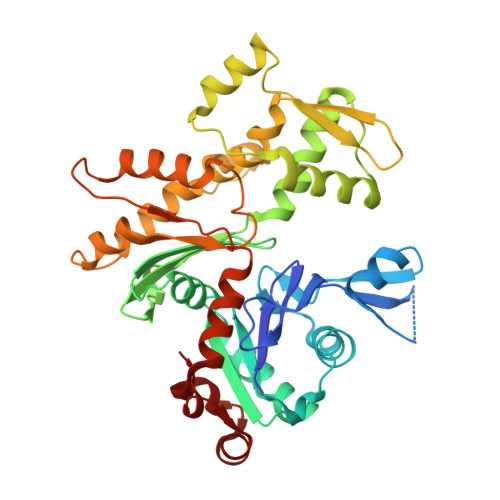
Connecting actin monomers by iso-peptide bond is a toxicity mechanism of the Vibrio cholerae MARTX toxin.
Kudryashov, D.S., Durer, Z.A., Ytterberg, A.J., Sawaya, M.R., Pashkov, I., Prochazkova, K., Yeates, T.O., Loo, R.R., Loo, J.A., Satchell, K.J., Reisler, E.(2008) Proc Natl Acad Sci U S A 105: 18537-18542
- PubMed: 19015515
- DOI: https://doi.org/10.1073/pnas.0808082105
- Primary Citation of Related Structures:
3CJB, 3CJC - PubMed Abstract:
The Gram-negative bacterium Vibrio cholerae is the causative agent of a severe diarrheal disease that afflicts three to five million persons annually, causing up to 200,000 deaths. Nearly all V. cholerae strains produce a large multifunctional-autoprocessing RTX toxin (MARTX(Vc)), which contributes significantly to the pathogenesis of cholera in model systems. The actin cross-linking domain (ACD) of MARTX(Vc) directly catalyzes a covalent cross-linking of monomeric G-actin into oligomeric chains and causes cell rounding, but the nature of the cross-linked bond and the mechanism of the actin cytoskeleton disruption remained elusive. To elucidate the mechanism of ACD action and effect on actin, we identified the covalent cross-link bond between actin protomers using limited proteolysis, X-ray crystallography, and mass spectrometry. We report here that ACD catalyzes the formation of an intermolecular iso-peptide bond between residues E270 and K50 located in the hydrophobic and the DNaseI-binding loops of actin, respectively. Mutagenesis studies confirm that no other residues on actin can be cross-linked by ACD both in vitro and in vivo. This cross-linking locks actin protomers into an orientation different from that of F-actin, resulting in strong inhibition of actin polymerization. This report describes a microbial toxin mechanism acting via iso-peptide bond cross-linking between host proteins and is, to the best of our knowledge, the only known example of a peptide linkage between nonterminal glutamate and lysine side chains.
- Department of Chemistry and Biochemistry and Molecular Biology Institute, University of California, Los Angeles, CA 90095, USA.
Organizational Affiliation: