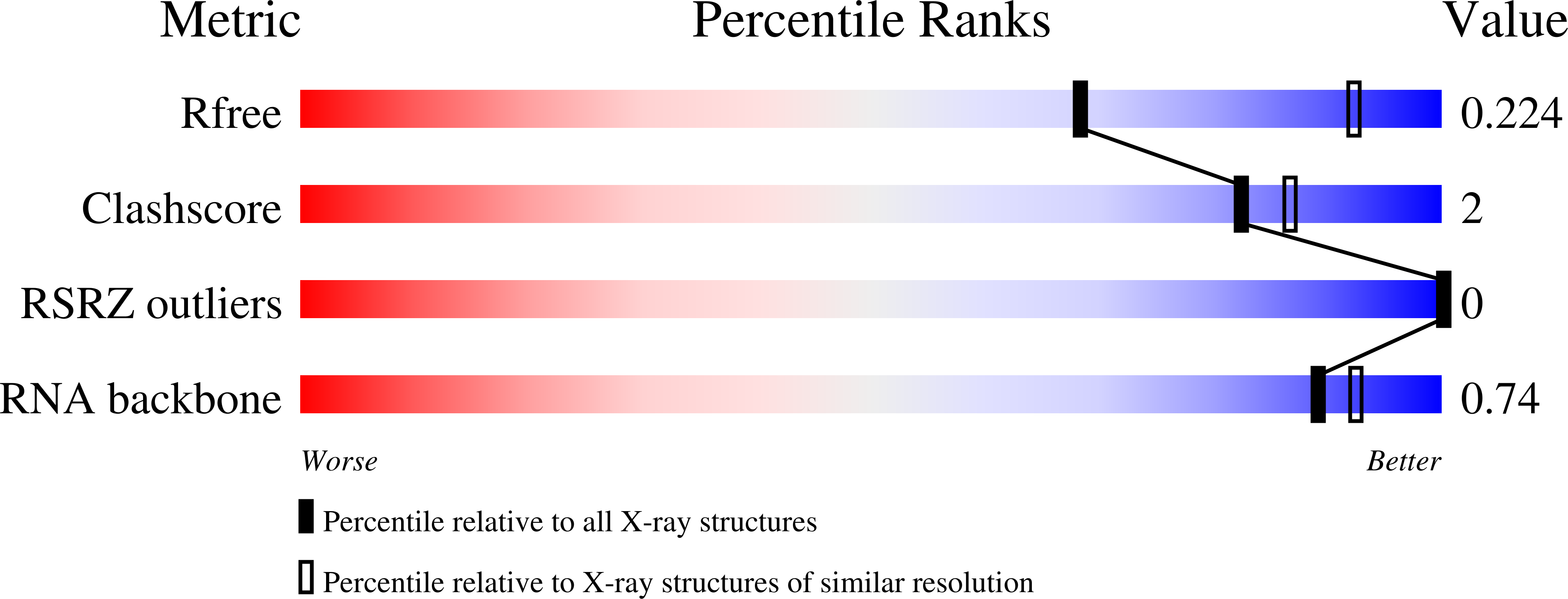X-ray structures of U2 snRNA-branchpoint duplexes containing conserved pseudouridines.
Lin, Y., Kielkopf, C.L.(2008) Biochemistry 47: 5503-5514
- PubMed: 18435545
- DOI: https://doi.org/10.1021/bi7022392
- Primary Citation of Related Structures:
3CGP, 3CGQ, 3CGR, 3CGS - PubMed Abstract:
A pseudouridine-modified region of the U2 small nuclear (sn)RNA anneals with the intronic branchpoint sequence and positions a bulged adenosine to serve as the nucleophile in the first chemical step of pre-mRNA splicing. We have determined three X-ray structures of RNA oligonucleotides containing the pseudouridylated U2 snRNA and the branchpoint consensus sequences. The expected adenosine branchpoint is extrahelical in a 1.65 A resolution structure containing the mammalian consensus sequence variant and in a 2.10 A resolution structure containing a shortened Saccharomyces cerevisiae consensus sequence. The adenosine adjacent to the expected branchpoint is extrahelical in a third structure, which contains the intact yeast consensus sequence at 1.57 A resolution. The hydration and base stacking interactions mediated by the U2 snRNA pseudouridines correlate with the identity of the unpaired adenosine. The expected adenosine bulge is associated with a well-stacked pseudouridine, which is linked via an ordered water molecule to a neighboring nucleotide. In contrast, the bulge of the adjacent adenosine shifts the base stacking and disrupts the water-mediated interactions of the pseudouridine. These structural differences may contribute to the ability of the pseudouridine modification to promote the bulged conformation of the branch site adenosine and to enhance catalysis by snRNAs. Furthermore, iodide binding sites are identified adjacent to the unconventional bulged adenosine, and the structure of the mammalian consensus sequence variant provides a high-resolution view of a hydrated magnesium ion bound in a similar manner to a divalent cation binding site of the group II intron.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland 21205, USA.