Involvement of a carboxylated lysine in UV damage endonuclease
Meulenbroek, E.M., Paspaleva, K., Thomassen, E.A., Abrahams, J.P., Goosen, N., Pannu, N.S.(2009) Protein Sci 18: 549-558
- PubMed: 19241382
- DOI: https://doi.org/10.1002/pro.54
- Primary Citation of Related Structures:
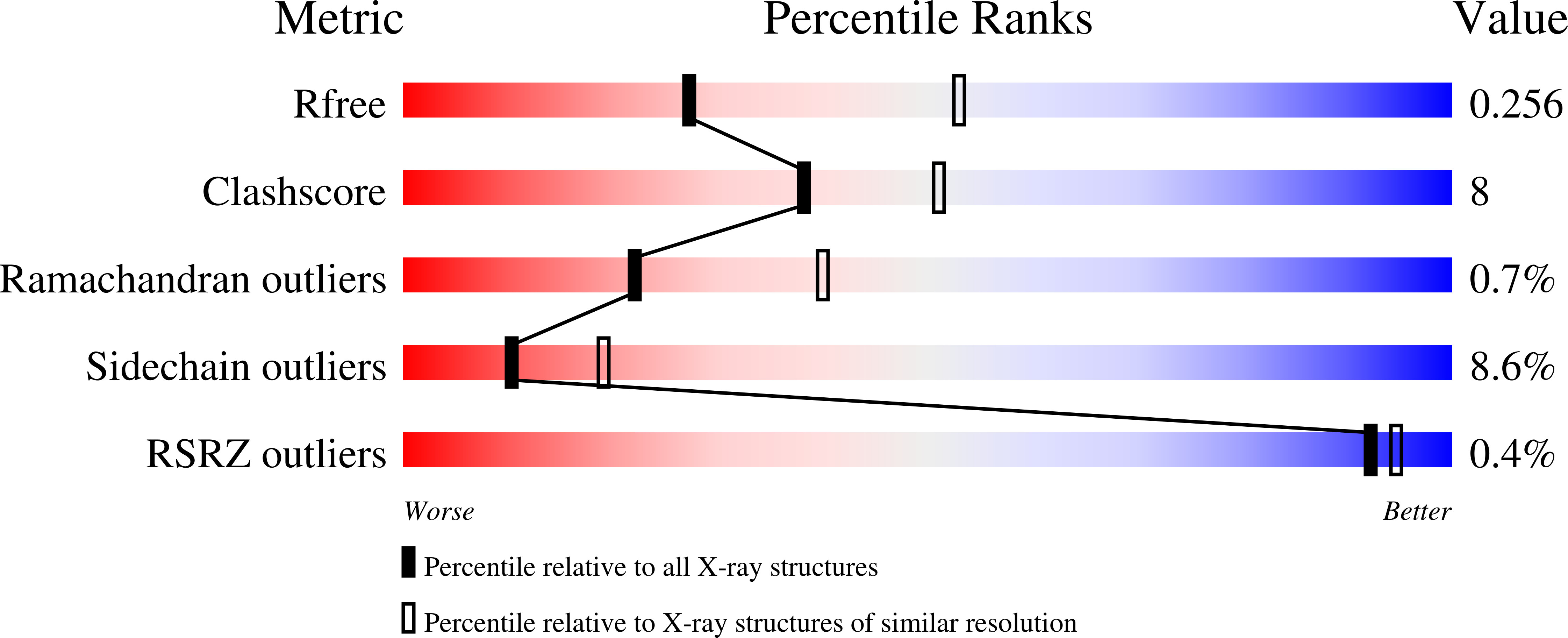
3BZG, 3BZJ, 3C0L, 3C0Q, 3C0S - PubMed Abstract:
UV damage endonuclease is a DNA repair enzyme that can both recognize damage such as UV lesions and introduce a nick directly 5' to them. Recently, the crystal structure of the enzyme from Thermus thermophilus was solved. In the electron density map of this structure, unexplained density near the active site was observed at the tip of Lys229. Based on this finding, it was proposed that Lys229 is post-translationally modified. In this article, we give evidence that this modification is a carboxyl group. By combining activity assays and X-ray crystallography on several point mutants, we show that the carboxyl group assists in metal binding required for catalysis by donating negative charge to the metal-coordinating residue His231. Moreover, functional and structural analysis of the K229R mutant reveals that if His231 shifts away, an increased activity results on both damaged and undamaged DNA. Taken together, the results show that T. thermophilus ultraviolet damage endonuclease is carboxylated and the modified lysine is required for proper catalysis and preventing increased incision of undamaged DNA.
Organizational Affiliation:
Biophysical Structural Chemistry, Leiden Institute of Chemistry, RA Leiden, The Netherlands.