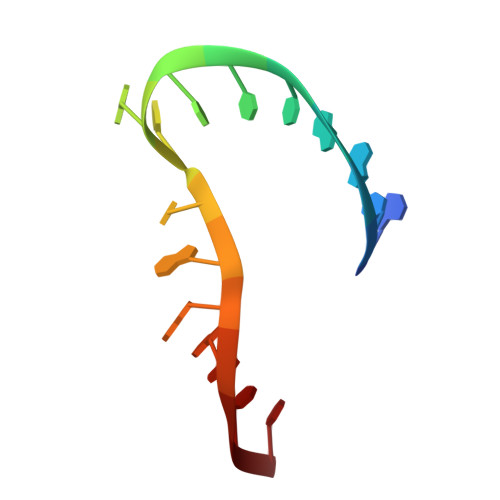
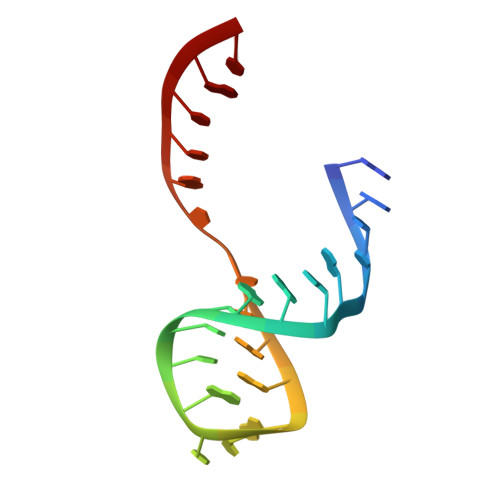Capturing the structure of a catalytic RNA intermediate: the hammerhead ribozyme.
Scott, W.G., Murray, J.B., Arnold, J.R., Stoddard, B.L., Klug, A.(1996) Science 274: 2065-2069
- PubMed: 8953035
- DOI: https://doi.org/10.1126/science.274.5295.2065
- Primary Citation of Related Structures:
299D, 300D, 301D - PubMed Abstract:
The crystal structure of an unmodified hammerhead RNA in the absence of divalent metal ions has been solved, and it was shown that this ribozyme can cleave itself in the crystal when divalent metal ions are added. This biologically active RNA fold is the same as that found previously for two modified hammerhead ribozymes. Addition of divalent cations at low pH makes it possible to capture the uncleaved RNA in metal-bound form. A conformational intermediate, having an additional Mg(II) bound to the cleavage-site phosphate, was captured by freeze-trapping the RNA at an active pH prior to cleavage. The most significant conformational changes were limited to the active site of the ribozyme, and the changed conformation requires only small additional movements to reach a proposed transition-state.
- MRC Laboratory of Molecular Biology, Hills Road, Cambridge CB2 2QH, England.
Organizational Affiliation: