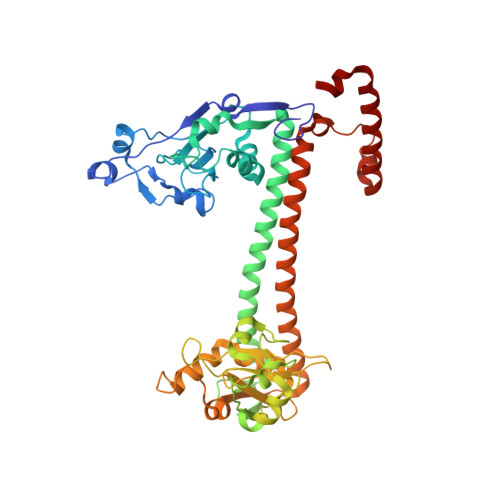
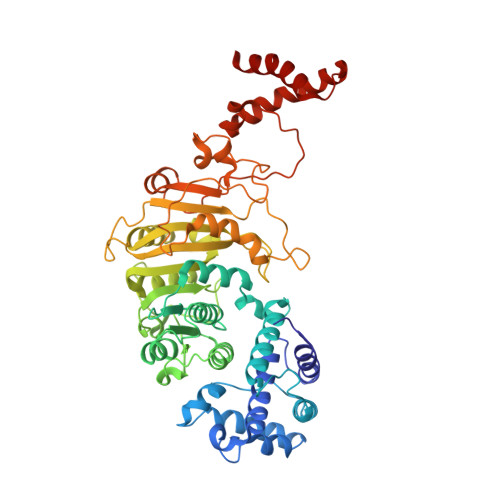
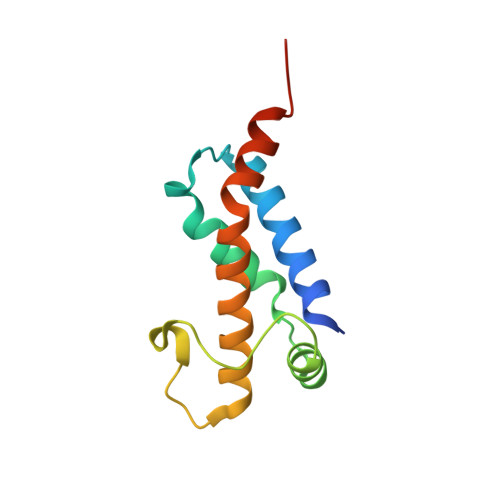
The Structure of M.Ecoki Type I DNA Methyltransferase with a DNA Mimic Antirestriction Protein.
Kennaway, C.K., Obarska-Kosinska, A., White, J.H., Tuszynska, I., Cooper, L.P., Bujnicki, J.M., Trinick, J., Dryden, D.T.F.(2009) Nucleic Acids Res 37: 762
- PubMed: 19074193
- DOI: https://doi.org/10.1093/nar/gkn988
- Primary Citation of Related Structures:
2Y7C, 2Y7H - PubMed Abstract:
Type-I DNA restriction-modification (R/M) systems are important agents in limiting the transmission of mobile genetic elements responsible for spreading bacterial resistance to antibiotics. EcoKI, a Type I R/M enzyme from Escherichia coli, acts by methylation- and sequence-specific recognition, leading to either methylation of DNA or translocation and cutting at a random site, often hundreds of base pairs away. Consisting of one specificity subunit, two modification subunits, and two DNA translocase/endonuclease subunits, EcoKI is inhibited by the T7 phage antirestriction protein ocr, a DNA mimic. We present a 3D density map generated by negative-stain electron microscopy and single particle analysis of the central core of the restriction complex, the M.EcoKI M(2)S(1) methyltransferase, bound to ocr. We also present complete atomic models of M.EcoKI in complex with ocr and its cognate DNA giving a clear picture of the overall clamp-like operation of the enzyme. The model is consistent with a large body of experimental data on EcoKI published over 40 years.
- Astbury Centre, Institute of Molecular and Cellular Biology, University of Leeds, UK.
Organizational Affiliation: