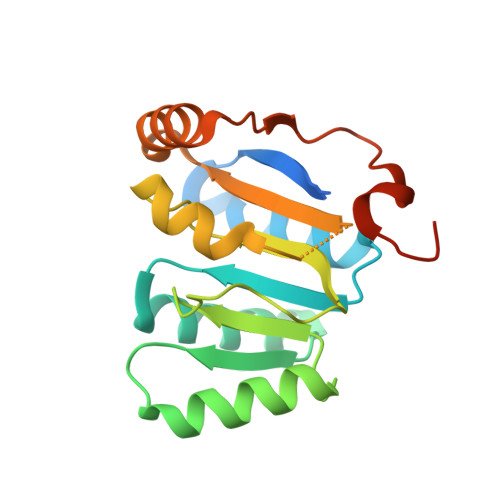
Structural Basis for the Mutually Exclusive Anchoring of P Body Components Edc3 and Tral to the Dead Box Protein Ddx6/Me31B.
Tritschler, F., Braun, J.E., Eulalio, A., Truffault, V., Izaurralde, E., Weichenrieder, O.(2009) Mol Cell 33: 661
- PubMed: 19285948
- DOI: https://doi.org/10.1016/j.molcel.2009.02.014
- Primary Citation of Related Structures:
2WAX, 2WAY - PubMed Abstract:
The DEAD box helicase DDX6/Me31B functions in translational repression and mRNA decapping. How particular RNA helicases are recruited specifically to distinct functional complexes is poorly understood. We present the crystal structure of the DDX6 C-terminal RecA-like domain bound to a highly conserved FDF sequence motif in the decapping activator EDC3. The FDF peptide adopts an alpha-helical conformation upon binding to DDX6, occupying a shallow groove opposite to the DDX6 surface involved in RNA binding and ATP hydrolysis. Mutagenesis of Me31B shows the relevance of the FDF interaction surface both for Me31B's accumulation in P bodies and for its ability to repress the expression of bound mRNAs. The translational repressor Tral contains a similar FDF motif. Together with mutational and competition studies, the structure reveals why the interactions of Me31B with EDC3 and Tral are mutually exclusive and how the respective decapping and translational repressor complexes might hook onto an mRNA substrate.
- Max Planck Institute for Developmental Biology, Tübingen, Germany.
Organizational Affiliation: