On the Disordered Activation Domain in Trypsinogen. Chemical Labelling and Low-Temperature Crystallography
Walter, J., Steigemann, W., Singh, T.P., Bartunik, H., Bode, W., Huber, R.(1982) Acta Crystallogr B 38: 1462-1472
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
(1982) Acta Crystallogr B 38: 1462-1472
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| TRYPSINOGEN | A [auth Z] | 229 | Bos taurus | Mutation(s): 0 Gene Names: PRSS1 EC: 3.4.21.4 | 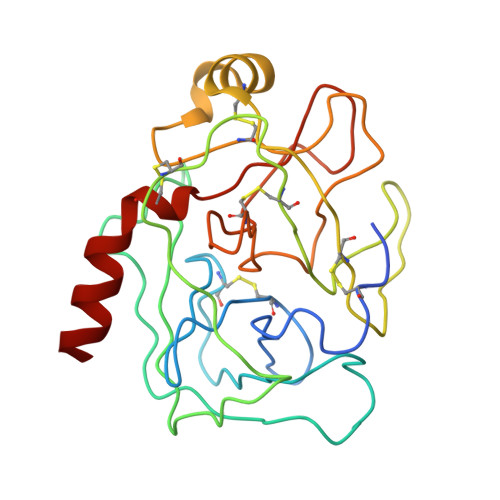 |
UniProt | |||||
Find proteins for P00760 (Bos taurus) Explore P00760 Go to UniProtKB: P00760 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00760 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| TRYPSIN INHIBITOR | B [auth I] | 58 | Bos taurus | Mutation(s): 0 | 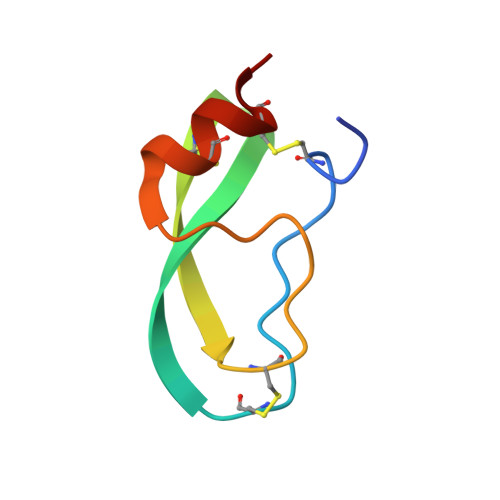 |
UniProt | |||||
Find proteins for P00974 (Bos taurus) Explore P00974 Go to UniProtKB: P00974 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00974 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| HG Query on HG | E [auth Z] | MERCURY (II) ION Hg BQPIGGFYSBELGY-UHFFFAOYSA-N |  | ||
| ILE Query on ILE | C [auth Z] | ISOLEUCINE C6 H13 N O2 AGPKZVBTJJNPAG-WHFBIAKZSA-N |  | ||
| VAL Query on VAL | D [auth Z] | VALINE C5 H11 N O2 KZSNJWFQEVHDMF-BYPYZUCNSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 75.67 | α = 90 |
| b = 84.16 | β = 90 |
| c = 122.89 | γ = 90 |