Crystal structure of the effector domain of PLXNB1 bound with Rnd1 GTPase.
Tong, Y., Tempel, W., Shen, L., Arrowsmith, C.H., Edwards, A.M., Sundstrom, M., Weigelt, J., Bochkarev, A., Park, H.To be published.
Experimental Data Snapshot
Starting Models: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Plexin-B1 | 121 | Homo sapiens | Mutation(s): 0 Gene Names: PLXNB1, KIAA0407, PLXN5, SEP | 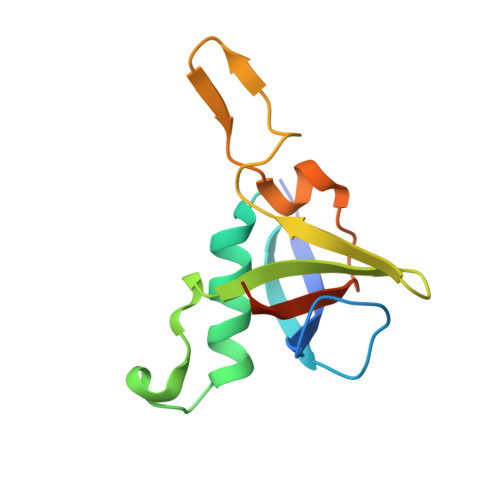 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O43157 (Homo sapiens) Explore O43157 Go to UniProtKB: O43157 | |||||
PHAROS: O43157 GTEx: ENSG00000164050 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O43157 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Rho-related GTP-binding protein Rho6 | 197 | Homo sapiens | Mutation(s): 0 Gene Names: RND1, RHO6 | 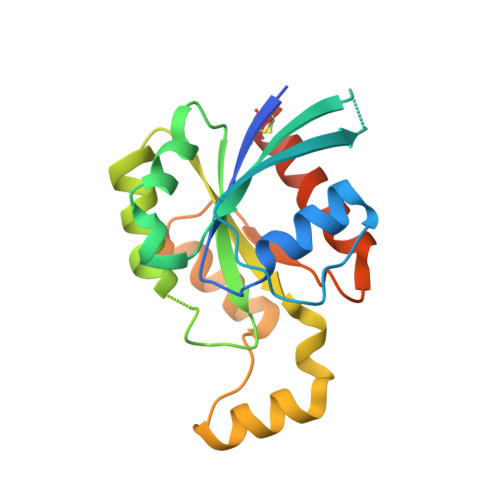 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q92730 (Homo sapiens) Explore Q92730 Go to UniProtKB: Q92730 | |||||
PHAROS: Q92730 GTEx: ENSG00000172602 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q92730 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 4 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| GNP Query on GNP | DA [auth D], K [auth B] | PHOSPHOAMINOPHOSPHONIC ACID-GUANYLATE ESTER C10 H17 N6 O13 P3 UQABYHGXWYXDTK-UUOKFMHZSA-N |  | ||
| CA Query on CA | CA [auth D], J [auth B] | CALCIUM ION Ca BHPQYMZQTOCNFJ-UHFFFAOYSA-N |  | ||
| MG Query on MG | BA [auth D], I [auth B] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| UNX Query on UNX | AA [auth C] E [auth A] EA [auth D] F [auth A] FA [auth D] | UNKNOWN ATOM OR ION X |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 150.136 | α = 90 |
| b = 71.653 | β = 128.36 |
| c = 101.885 | γ = 90 |
| Software Name | Purpose |
|---|---|
| DENZO | data reduction |
| SCALEPACK | data scaling |
| PHASER | phasing |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| ADSC | data collection |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |