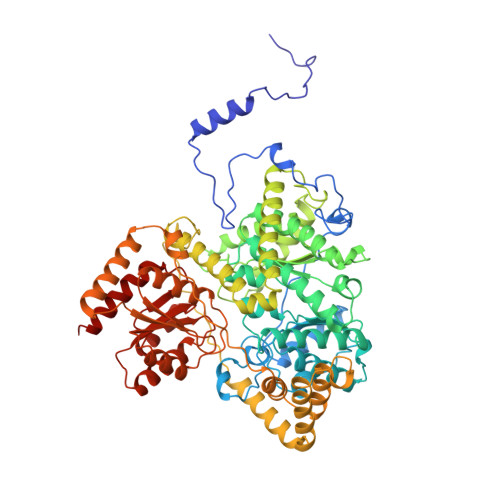
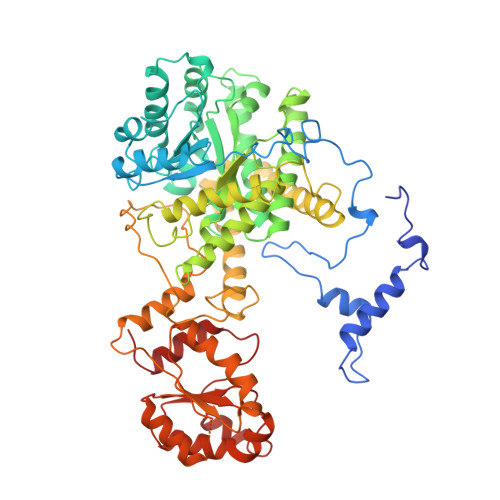
Conformational changes on substrate binding to methylmalonyl CoA mutase and new insights into the free radical mechanism.
Mancia, F., Evans, P.R.(1998) Structure 6: 711-720
- PubMed: 9655823
- DOI: https://doi.org/10.1016/s0969-2126(98)00073-2
- Primary Citation of Related Structures:
2REQ, 3REQ, 4REQ - PubMed Abstract:
Methylmalonyl CoA mutase catalyses the interconversion of succinyl CoA and methylmalonyl CoA via a free radical mechanism. The enzyme belongs to a family of enzymes that catalyse intramolecular rearrangement reactions in which a group and a hydrogen atom on adjacent carbons are exchanged. These enzymes use the cofactor adenosylcobalamin (coenzyme B12) which breaks to form an adenosyl radical, thus initiating the reaction. Determination of the structure of substrate-free methylmalonyl CoA mutase was initiated to provide further insight into the mechanism of radical formation. We report here two structures of methylmalonyl CoA mutase from Propionibacterium shermanii. The first structure is of the enzyme in a nonproductive complex with CoA at 2.5 A resolution. This structure serves as a model for the substrate-free conformation of the enzyme, as it is very similar to the second much poorer 2.7 A resolution structure derived from a truly substrate-free crystal. The true substrate-free structure also shows the adenosyl group bound to the cobalt atom. Comparison of this structure with that of the previously reported complex of the enzyme with a substrate analogue shows that major conformational changes occur upon substrate binding. The substrate-binding site of the enzyme is located within a (beta alpha)8 TIM-barrel domain. In the absence of substrate, this TIM-barrel domain is split apart and the active site is accessible to solvent. When substrate binds, the barrel closes up with the substrate along its axis and the active site becomes completely buried. The closure of the active-site cavity upon substrate binding displaces the adenosyl group of the cofactor from the central cobalt atom into the active-site cavity. This triggers the formation of the free radical that initiates the rearrangement reaction. The TIM-barrel domain is substantially different from all others yet reported: in its unliganded form it is broken open, exposing the small hydrophilic sidechains which fill the centre. The typical barrel structure is only formed when substrate is bound.
- MRC Laboratory of Molecular Biology Hills Road, Cambridge, CB2 2QH, UK.
Organizational Affiliation: