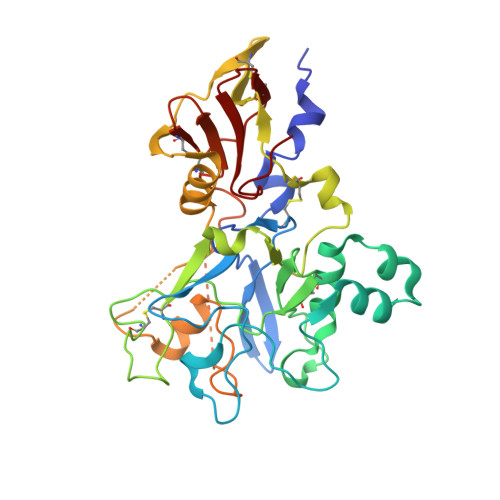
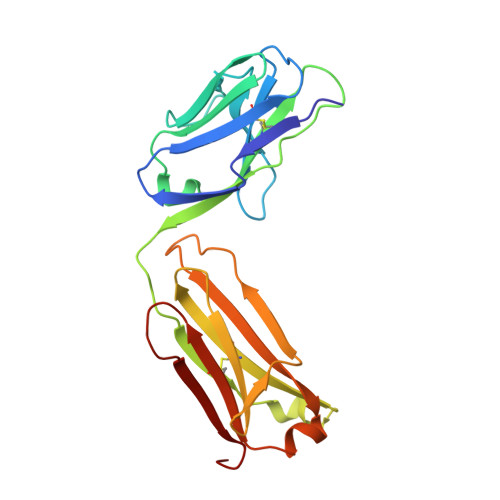
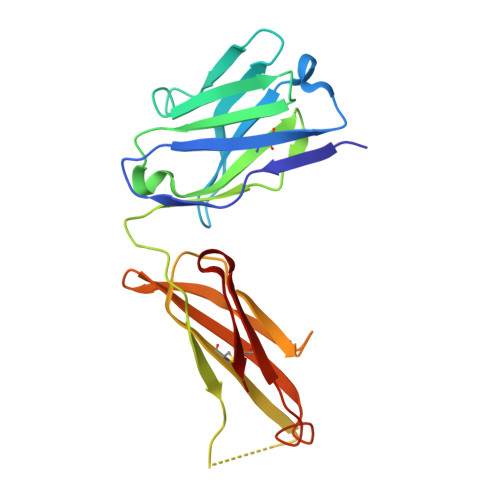
Structure of the malaria antigen AMA1 in complex with a growth-inhibitory antibody.
Coley, A.M., Gupta, A., Murphy, V.J., Bai, T., Kim, H., Foley, M., Anders, R.F., Batchelor, A.H.(2007) PLoS Pathog 3: 1308-1319
- PubMed: 17907804
- DOI: https://doi.org/10.1371/journal.ppat.0030138
- Primary Citation of Related Structures:
2Q8A, 2Q8B - PubMed Abstract:
Identifying functionally critical regions of the malaria antigen AMA1 (apical membrane antigen 1) is necessary to understand the significance of the polymorphisms within this antigen for vaccine development. The crystal structure of AMA1 in complex with the Fab fragment of inhibitory monoclonal antibody 1F9 reveals that 1F9 binds to the AMA1 solvent-exposed hydrophobic trough, confirming its importance. 1F9 uses the heavy and light chain complementarity-determining regions (CDRs) to wrap around the polymorphic loops adjacent to the trough, but uses a ridge of framework residues to bind to the hydrophobic trough. The resulting 1F9-AMA1-combined buried surface of 2,470 A(2) is considerably larger than previously reported Fab-antigen interfaces. Mutations of polymorphic AMA1 residues within the 1F9 epitope disrupt 1F9 binding and dramatically reduce the binding of affinity-purified human antibodies. Moreover, 1F9 binding to AMA1 is competed by naturally acquired human antibodies, confirming that the 1F9 epitope is a frequent target of immunological attack.
- Cooperative Research Center for Diagnostics, Department of Biochemistry, La Trobe University, Victoria, Australia.
Organizational Affiliation: