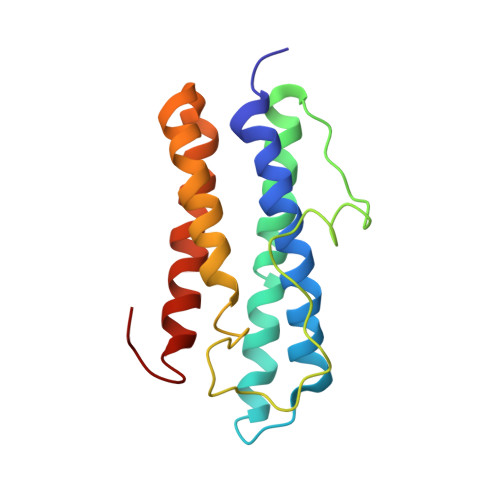
Structure and immunomodulatory property relationship in NapA of Borrelia burgdorferi.
Codolo, G., Papinutto, E., Polenghi, A., D'Elios, M.M., Zanotti, G., de Bernard, M.(2010) Biochim Biophys Acta 1804: 2191-2197
- PubMed: 20851780
- DOI: https://doi.org/10.1016/j.bbapap.2010.09.004
- Primary Citation of Related Structures:
2PYB - PubMed Abstract:
NapA from Borrelia burgdorferi is a member of the Dps-like protein family with specific immunomodulatory properties; in particular, NapA is able to induce the expression of IL-23 in neutrophils and monocytes, as well as the expression of IL-6, IL-1β, and transforming growth factor beta (TGF-β) in monocytes, via Toll-like receptor (TLR) 2. Such an activity on innate immune cells triggers a synovial fluid Th17 response. Here we report the crystal structure of NapA, determined at 2.6Å resolution, which shows that the quaternary structure of the protein is that of a dodecamer with 23 symmetry, typical of the proteins of the family. We also demonstrate that the N- and C-terminal tails, which are flexible and not visible in the crystal, are not relevant for its pro-Th17 activity. Based on the crystal structure and on the comparison with the structure of the orthologous protein from Helicobacter pylori, HP-NAP, we hypothesize that the charge distributions on the two proteins' surfaces are responsible for the interaction with TLR2 and for the different behaviors in modulating the immune response.
- Venetian Institute of Molecular Medicine, Via Orus 2, 35129 Padua, Italy.
Organizational Affiliation: