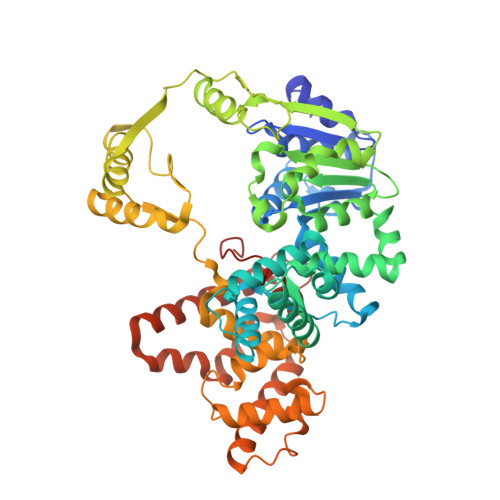
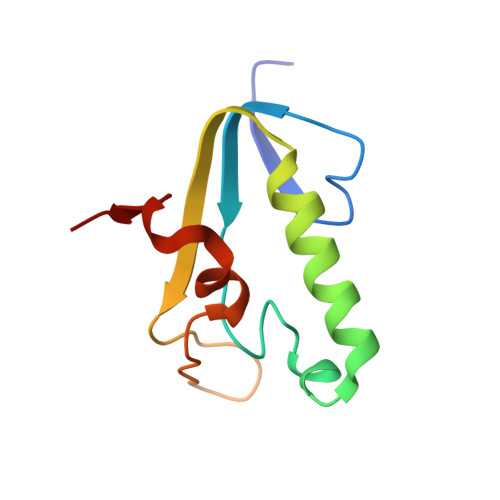
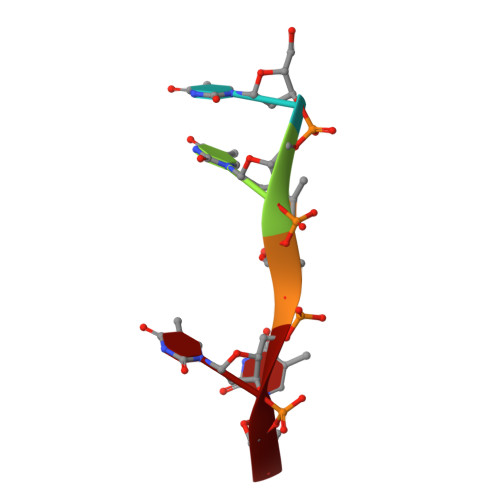
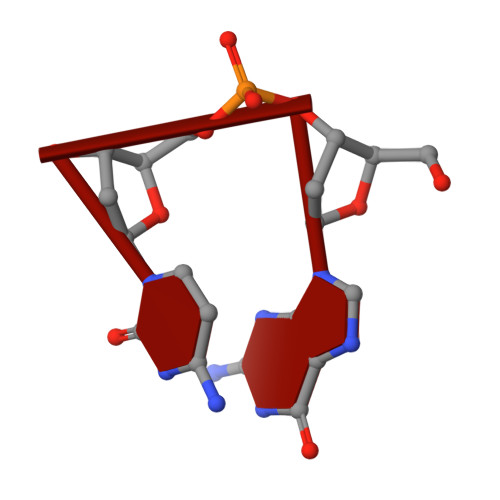
Crystal structures of complexes of PcrA DNA helicase with a DNA substrate indicate an inchworm mechanism.
Velankar, S.S., Soultanas, P., Dillingham, M.S., Subramanya, H.S., Wigley, D.B.(1999) Cell 97: 75-98
- PubMed: 10199404
- DOI: https://doi.org/10.1016/s0092-8674(00)80716-3
- Primary Citation of Related Structures:
2PJR, 3PJR - PubMed Abstract:
We have determined two different structures of PcrA DNA helicase complexed with the same single strand tailed DNA duplex, providing snapshots of different steps on the catalytic pathway. One of the structures is of a complex with a nonhydrolyzable analog of ATP and is thus a "substrate" complex. The other structure contains a bound sulphate ion that sits in a position equivalent to that occupied by the phosphate ion produced after ATP hydrolysis, thereby mimicking a "product" complex. In both complexes, the protein is monomeric. Large and distinct conformational changes occur on binding DNA and the nucleotide cofactor. Taken together, these structures provide evidence against an "active rolling" model for helicase action but are instead consistent with an "inchworm" mechanism.
- Sir William Dunn School of Pathology, University of Oxford, United Kingdom.
Organizational Affiliation: