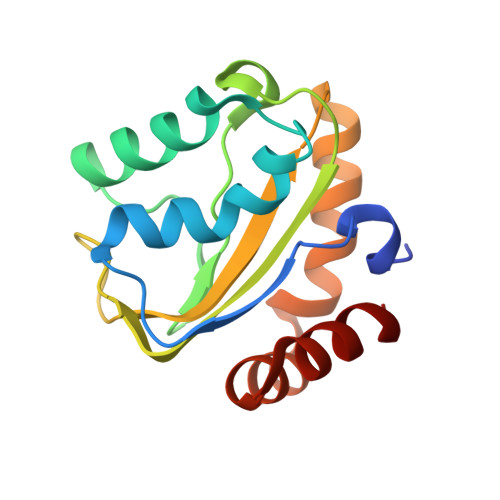
Solution structure of the cryptic mannitol-specific phosphotransferase enzyme IIA CmtB from Escherichia coli
Yu, C., Li, Y., Xia, B., Jin, C.(2007) Biochem Biophys Res Commun 362: 1001-1006
- PubMed: 17803963
- DOI: https://doi.org/10.1016/j.bbrc.2007.08.102
- Primary Citation of Related Structures:
2OQ3 - PubMed Abstract:
The bacterial phosphoenolpyruvate-dependent sugar phosphotransferase system (PEP-PTS) is essential in the coupled transportation and phosphorylation of various types of carbohydrates. The CmtAB proteins of Escherichia coli are sequentially similar to the mannitol-specific phosphotransferase MtlA. The CmtB protein corresponds to the phosphotransferase enzyme IIA component. Here we report the solution structure of CmtB from E. coli at high resolution by NMR spectroscopy. The results show that CmtB adopts a globular fold consisting of a central mixed five-strand beta-sheet flanked by seven helices at both sides. Structural comparison with the IIA domain of MtlA (IIAMtl) reveals high overall similarity, while notable conformational differences at the active site are observed. The active site pocket of CmtB appears to be wider, and the hydrophobic regions around it is larger compared to IIAMtl. Further, the essential arginine residue at the active site of IIAMtl is substituted by a serine in CmtB. Instead, the active pocket of CmtB contains another arginine at a distinct position, suggesting different molecular mechanisms for phosphoryl transfer.
- Beijing Nuclear Magnetic Resonance Center, Peking University, Beijing 100871, China.
Organizational Affiliation: