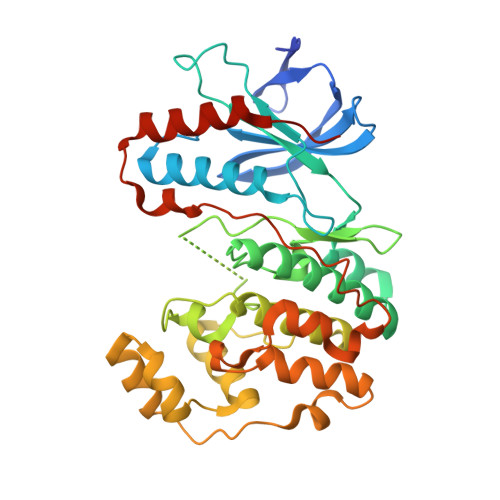
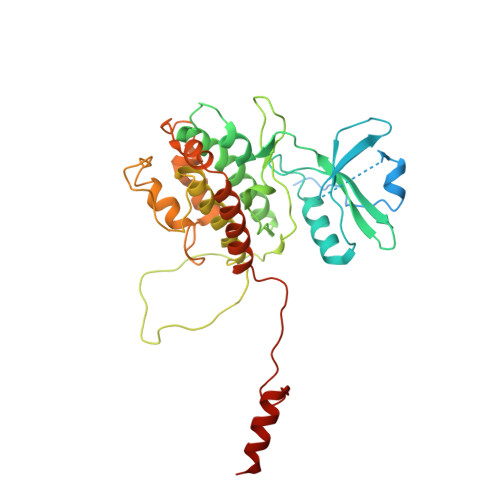
Crystal structure of the P38alpha-MAPKAP kinase 2 heterodimer.
ter Haar, E., Prabakhar, P., Liu, X., Lepre, C.(2007) J Biological Chem 282: 9733-9739
- PubMed: 17255097
- DOI: https://doi.org/10.1074/jbc.M611165200
- Primary Citation of Related Structures:
2OKR, 2ONL - PubMed Abstract:
The p38 signaling pathway is activated in response to cell stress and induces production of proinflammatory cytokines. P38alpha is phosphorylated and activated in response to cell stress by MKK3 and MKK6 and in turn phosphorylates a number of substrates, including MAPKAP kinase 2 (MK2). We have determined the crystal structure of the unphosphorylated p38alpha-MK2 heterodimer. The C-terminal regulatory domain of MK2 binds in the docking groove of p38alpha, and the ATP-binding sites of both kinases are at the heterodimer interface. The conformation suggests an extra mechanism in addition to the regulation of the p38alpha and MK2 phosphorylation states that prevents phosphorylation of substrates in the absence of cell stress. Addition of constitutively active MKK6-DD results in rapid phosphorylation of the p38alpha-MK2 heterodimer.
- Vertex Pharmaceuticals Incorporated, Cambridge, Massachusetts 02139. Electronic address: ernst_terhaar@vrtx.com.
Organizational Affiliation: