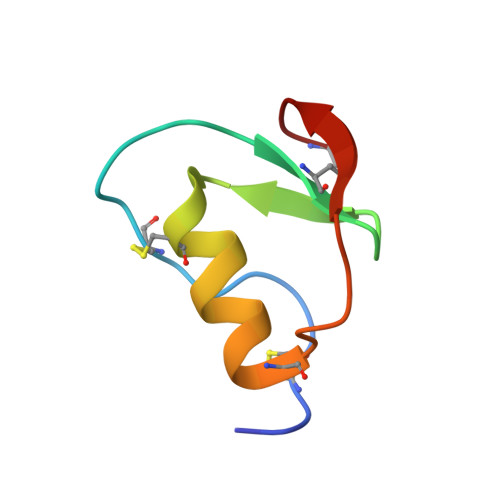
NMR structure of CmPI-II, a non-classical Kazal protease inhibitor: Understanding its conformational dynamics and subtilisin A inhibition.
Cabrera-Munoz, A., Valiente, P.A., Rojas, L., Alonso-Del-Rivero Antigua, M., Pires, J.R.(2019) J Struct Biol 206: 280-294
- PubMed: 30930219
- DOI: https://doi.org/10.1016/j.jsb.2019.03.011
- Primary Citation of Related Structures:
2N71 - PubMed Abstract:
Subtilisin-like proteases play crucial roles in host-pathogen interactions. Thus, protease inhibitors constitute important tools in the regulation of this interaction. CmPI-II is a Kazal proteinase inhibitor isolated from Cenchritis muricatus that inhibits subtilisin A, trypsin and elastases. Based on sequence analysis it defines a new group of non-classical Kazal inhibitors. Lacking solved 3D structures from this group prevents the straightforward structural comparison with other Kazal inhibitors. The 3D structure of CmPI-II, solved in this work using NMR techniques, shows the typical fold of Kazal inhibitors, but has significant differences in its N-terminal moiety, the disposition of the CysI-CysV disulfide bond and the reactive site loop (RSL) conformation. The high flexibility of its N-terminal region, the RSL, and the α-helix observed in NMR experiments and molecular dynamics simulations, suggest a coupled motion of these regions that could explain CmPI-II broad specificity. The 3D structure of the CmPI-II/subtilisin A complex, obtained by modeling, allows understanding of the energetic basis of the subtilisin A inhibition. The residues at the P2 and P2' positions of the inhibitor RSL were predicted to be major contributors to the binding free energy of the complex, rather than those at the P1 position. Site directed mutagenesis experiments confirmed the Trp14 (P2') contribution to CmPI-II/subtilisin A complex formation. Overall, this work provides the structural determinants for the subtilisin A inhibition by CmPI-II and allows the designing of more specific and potent molecules. In addition, the 3D structure obtained supports the existence of a new group in non-classical Kazal inhibitors.
- Centro de Estudios de Proteínas, Facultad de Biología, Universidad de La Habana, La Habana-Cuba, Calle 25 No 455, Vedado, La Habana, Cuba. Electronic address: aymara@fbio.uh.cu.
Organizational Affiliation: