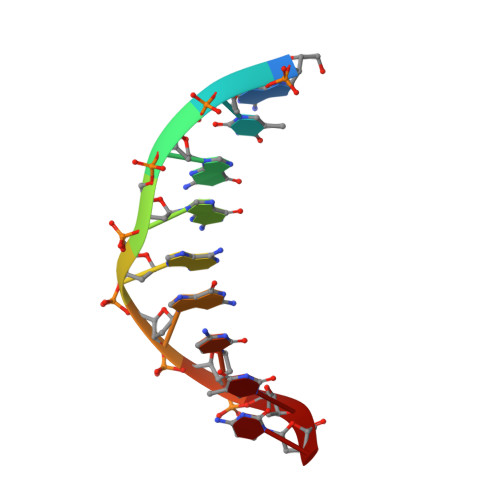
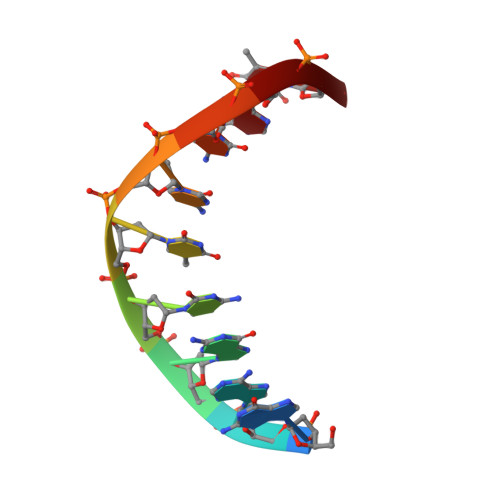
Using NMR and molecular dynamics to link structure and dynamics effects of the universal base 8-aza, 7-deaza, N8 linked adenosine analog.
Spring-Connell, A.M., Evich, M.G., Debelak, H., Seela, F., Germann, M.W.(2016) Nucleic Acids Res 44: 8576-8587
- PubMed: 27566150
- DOI: https://doi.org/10.1093/nar/gkw736
- Primary Citation of Related Structures:
2N5O, 2N5P - PubMed Abstract:
A truly universal nucleobase enables a host of novel applications such as simplified templates for PCR primers, randomized sequencing and DNA based devices. A universal base must pair indiscriminately to each of the canonical bases with little or preferably no destabilization of the overall duplex. In reality, many candidates either destabilize the duplex or do not base pair indiscriminatingly. The novel base 8-aza-7-deazaadenine (pyrazolo[3,4-d]pyrimidin- 4-amine) N 8 -(2'deoxyribonucleoside), a deoxyadenosine analog (UB), pairs with each of the natural DNA bases with little sequence preference. We have utilized NMR complemented with molecular dynamic calculations to characterize the structure and dynamics of a UB incorporated into a DNA duplex. The UB participates in base stacking with little to no perturbation of the local structure yet forms an unusual base pair that samples multiple conformations. These local dynamics result in the complete disappearance of a single UB proton resonance under native conditions. Accommodation of the UB is additionally stabilized via heightened backbone conformational sampling. NMR combined with various computational techniques has allowed for a comprehensive characterization of both structural and dynamic effects of the UB in a DNA duplex and underlines that the UB as a strong candidate for universal base applications.
- Department of Chemistry, Georgia State University, Atlanta, GA 30303, USA.
Organizational Affiliation: