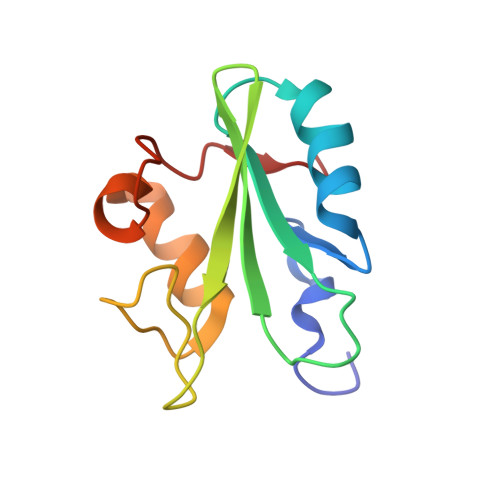
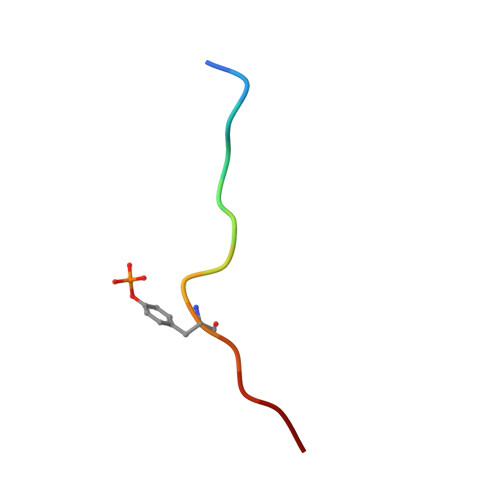
Differential recognition of syk-binding sites by each of the two phosphotyrosine-binding pockets of the Vav SH2 domain.
Chen, C.H., Piraner, D., Gorenstein, N.M., Geahlen, R.L., Beth Post, C.(2013) Biopolymers 99: 897-907
- PubMed: 23955592
- DOI: https://doi.org/10.1002/bip.22371
- Primary Citation of Related Structures:
2MC1 - PubMed Abstract:
The association of spleen tyrosine kinase (Syk), a central tyrosine kinase in B cell signaling, with Vav SH2 domain is controlled by phosphorylation of two closely spaced tyrosines in Syk linker B: Y342 and Y346. Previous studies established both singly phosphorylated and doubly phosphorylated forms play a role in signaling. The structure of the doubly phosphorylated form identified a new recognition of phosphotyrosine whereby two phosphotyrosines bind simultaneously to the Vav SH2 domain, one in the canonical pTyr pocket and one in the specificity pocket on the opposite side of the central β-sheet. It is unknown if the specificity pocket can bind phosphotyrosine independent of phosphotyrosine binding the pTyr pocket. To address this gap in knowledge, we determined the structure of the complex between Vav1 SH2 and a peptide (SykLB-YpY) modeling the singly phosphorylated-Y346 form of Syk with unphosphorylated Y342. The nuclear magnetic resonance (NMR) data conclusively establish that recognition of phosphotyrosine is swapped between the two pockets; phosphorylated pY346 binds the specificity pocket of Vav1 SH2, and unphosphorylated Y342 occupies what is normally the pTyr binding pocket. Nearly identical changes in chemical shifts occurred upon binding all three forms of singly and doubly phosphorylated peptides; however, somewhat smaller shift perturbations for SykLB-YpY from residues in regions of high internal mobility suggest that internal motions are coupled to binding affinity. The differential recognition that includes this swapped binding of phosphotyrosine to the specificity pocket of Vav SH2 increases the repertoire of possible phosphotyrosine binding by SH2 domains in regulating protein-protein interactions in cellular signaling.
- Department of Medicinal Chemistry and Molecular Pharmacology, and Purdue Center for Cancer Research, Purdue University, West Lafayette, IN, 47907.
Organizational Affiliation: