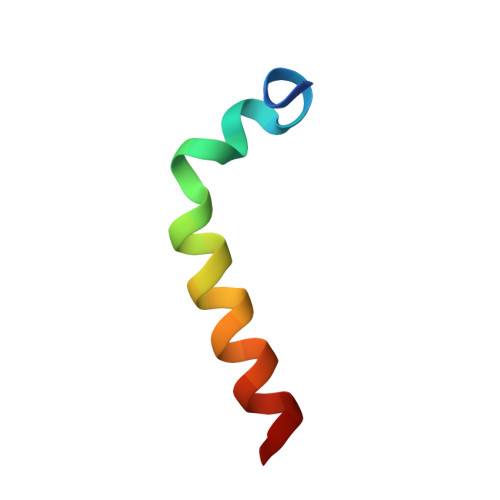
NMR structure of human thymosin alpha-1.
Elizondo-Riojas, M.A., Chamow, S.M., Tuthill, C.W., Gorenstein, D.G., Volk, D.E.(2011) Biochem Biophys Res Commun 416: 356-361
- PubMed: 22115779
- DOI: https://doi.org/10.1016/j.bbrc.2011.11.041
- Primary Citation of Related Structures:
2L9I - PubMed Abstract:
800 MHz NMR structure of the 28-residue peptide thymosin alpha-1 in 40% TFE/60% water (v/v) has been determined. Restrained molecular dynamic simulations with an explicit solvent box containing 40% TFE/60% TIP3P water (v/v) were used, in order to get the 3D model of the NMR structure. We found that the peptide adopts a structured conformation having two stable regions: an alpha-helix region from residues 14 to 26 and two double β-turns in the N-terminal twelve residues which form a distorted helical structure.
- Center for Proteomics and Systems Biology, Institute of Molecular Medicine for Prevention of Human Diseases, Department of NanoMedicine and Biomedical Engineering, University of Texas Health Science Center-Houston, 1825 Pressler, Houston, TX 77030, United States.
Organizational Affiliation: