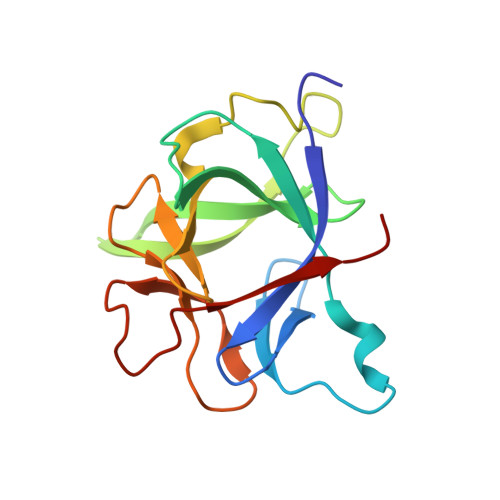
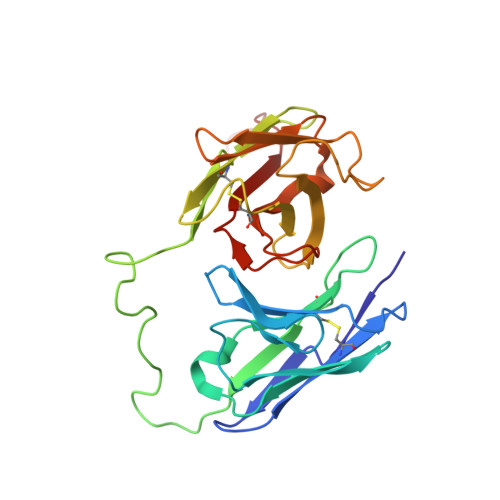
High resolution NMR-based model for the structure of a scFv-IL-1beta complex: potential for NMR as a key tool in therapeutic antibody design and development.
Wilkinson, I.C., Hall, C.J., Veverka, V., Shi, J.Y., Muskett, F.W., Stephens, P.E., Taylor, R.J., Henry, A.J., Carr, M.D.(2009) J Biological Chem 284: 31928-31935
- PubMed: 19776018
- DOI: https://doi.org/10.1074/jbc.M109.025304
- Primary Citation of Related Structures:
2KH2 - PubMed Abstract:
Monoclonal antibodies have recently started to deliver on their promise as highly specific and active drugs; however, a more effective, knowledge-based approach to the selection, design, and optimization of potential therapeutic antibodies is currently limited by the surprising lack of detailed structural information for complexes formed with target proteins. Here we show that complexes formed with minimal antigen binding single chain variable fragments (scFv) reliably reflect all the features of the binding interface present in larger Fab fragments, which are commonly used as therapeutics, and report the development of a robust, reliable, and relatively rapid approach to the determination of high resolution models for scFv-target protein complexes. This NMR spectroscopy-based approach combines experimental determination of the interaction surfaces and relative orientations of the scFv and target protein, with NMR restraint-driven, semiflexible docking of the proteins to produce a reliable and highly informative model of the complex. Experience with scFvs and Fabs targeted at a number of secreted regulatory proteins suggests that the approach will be applicable to many therapeutic antibodies targeted at proteins, and its application is illustrated for a potential therapeutic antibody targeted at the cytokine IL-1beta. The detailed structural information that can be obtained by this approach has the potential to have a major impact on the rational design and development of an increasingly important class of biological pharmaceuticals.
- Department of Biochemistry, University of Leicester, Leicester LE1 9HN, United Kingdom.
Organizational Affiliation: