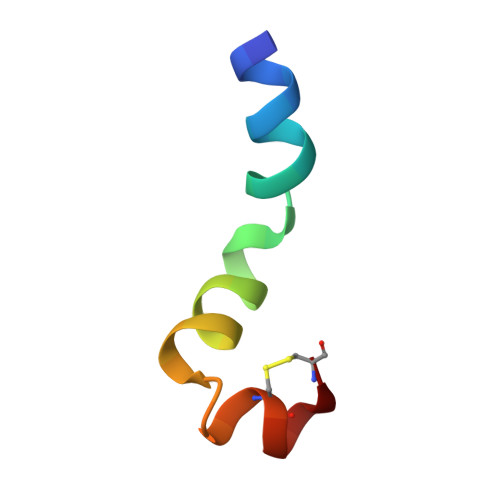
Conformational analysis of the broad-spectrum antibacterial peptide, ranatuerin-2CSa: Identification of a full length helix-turn-helix motif.
Subasinghage, A.P., Conlon, J.M., Hewage, C.M.(2008) Biochim Biophys Acta 1784: 924-929
- PubMed: 18387372
- DOI: https://doi.org/10.1016/j.bbapap.2008.02.019
- Primary Citation of Related Structures:
2K10 - PubMed Abstract:
Design of clinically valuable antibacterial agents based upon naturally occurring peptides requires the use of spectroscopic methods, particularly NMR, to determine the three-dimensional structure of the native peptide so that analogues with improved therapeutic properties can be made. Ranatuerin-2CSa (GILSSFKGVAKGVAKDLAG KLLETLKCKITGC), first isolated from skin secretions of the Cascades frog, Rana cascadae, represents a promising candidate for drug development. The peptide shows potent growth inhibitory activity against Escherichia coli (MIC=5 microM) and Staphylococcus aureus (MIC=10 microM) but displays haemolytic activity against human erythrocytes (LC(50)=160 microM). The solution structure of ranatuerin-2CSa was investigated by proton NMR spectroscopy and molecular modelling. In aqueous solution, the peptide lacks secondary structure but, in a 2,2,2-trifluoroethanol (TFE-d(3))-H(2)O solvent mixture, the structure is characterised by a full length helix-turn-helix conformation between residues I(2)-L(21), L(22)-L(25) and K(26)-T(30) respectively. This structural information will facilitate the design of novel therapeutic agents based upon the ranatuerin-2CSa structure with improved antimicrobial potencies but decreased cytolytic activities against mammalian cells.
- UCD School of Biomolecular and Biomedical Science, UCD Centre for Synthesis and Chemical Biology, UCD Conway institute, University College Dublin, Belfield, Dublin 4, Ireland.
Organizational Affiliation: