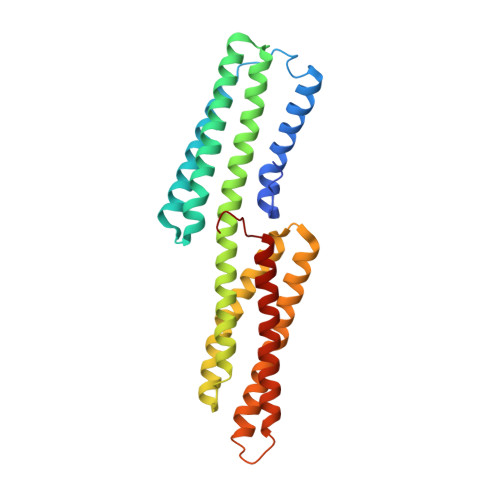
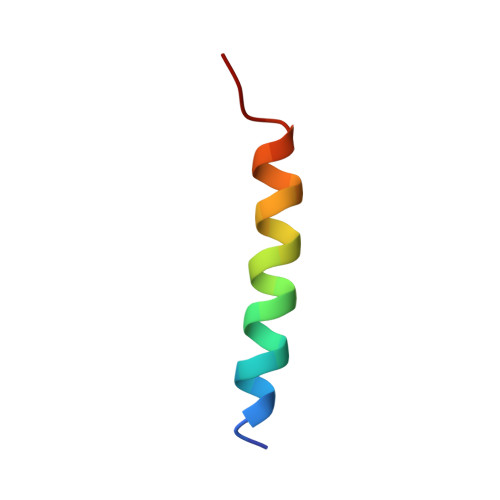
Vinculin binding in its closed conformation by a helix addition mechanism.
Nhieu, G.T., Izard, T.(2007) EMBO J 26: 4588-4596
- PubMed: 17932491
- DOI: https://doi.org/10.1038/sj.emboj.7601863
- Primary Citation of Related Structures:
2IBF - PubMed Abstract:
Vinculin links integrin receptors to the actin cytoskeleton by binding to talin. Vinculin is held in an inactive, closed-clamp conformation through hydrophobic interactions between its head and tail domains, and vinculin activation has long been thought to be dependent upon severing the head-tail interaction. Talin, alpha-actinin, and the invasin IpaA of Shigella flexneri sever vinculin's head-tail interaction by inserting an alpha-helix into vinculin's N-terminal four-helical bundle, provoking extensive conformational changes by a helical bundle conversion mechanism; these alterations in vinculin structure displace its tail domain, allowing vinculin to bind to its other partners. IpaA harbors two juxtaposed alpha-helical vinculin-binding sites (VBS) in its C-terminus. Here, we report that the lower affinity VBS of IpaA can also bind to the adjacent C-terminal four-helical bundle of vinculin's head domain through a helix addition mechanism. These hydrophobic interactions do not alter the conformation of this helical bundle, and the architecture of the complex suggests that IpaA can simultaneously interact with both of the four-helical bundle domains of vinculin's N-terminus to stabilize vinculin-IpaA interactions.
- Unité de Pathogénie Microbienne Moléculaire, Institut Pasteur, Inserm U786, Paris, France.
Organizational Affiliation: