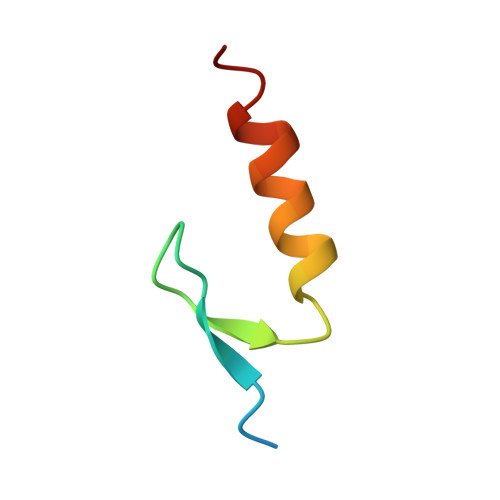
Structure of the ubiquitin-binding zinc finger domain of human DNA Y-polymerase eta.
Bomar, M.G., Pai, M.T., Tzeng, S.R., Li, S.S., Zhou, P.(2007) EMBO Rep 8: 247-251
- PubMed: 17304240
- DOI: https://doi.org/10.1038/sj.embor.7400901
- Primary Citation of Related Structures:
2I5O - PubMed Abstract:
The ubiquitin-binding zinc finger (UBZ) domain of human DNA Y-family polymerase (pol) eta is important in the recruitment of the polymerase to the stalled replication machinery in translesion synthesis. Here, we report the solution structure of the pol eta UBZ domain and its interaction with ubiquitin. We show that the UBZ domain adopts a classical C(2)H(2) zinc-finger structure characterized by a betabetaalpha fold. Nuclear magnetic resonance titration maps the binding interfaces between UBZ and ubiquitin to the alpha-helix of the UBZ domain and the canonical hydrophobic surface of ubiquitin defined by residues L8, I44 and V70. Although the UBZ domain binds ubiquitin through a single alpha-helix, in a manner similar to the inverted ubiquitin-interacting motif, its structure is distinct from previously characterized ubiquitin-binding domains. The pol eta UBZ domain represents a novel member of the C(2)H(2) zinc finger family that interacts with ubiquitin to regulate translesion synthesis.
- Department of Biochemistry, Duke University Medical Center, Research Drive, 242 Nanaline Duke Building, Durham, North Carolina 27710, USA.
Organizational Affiliation: