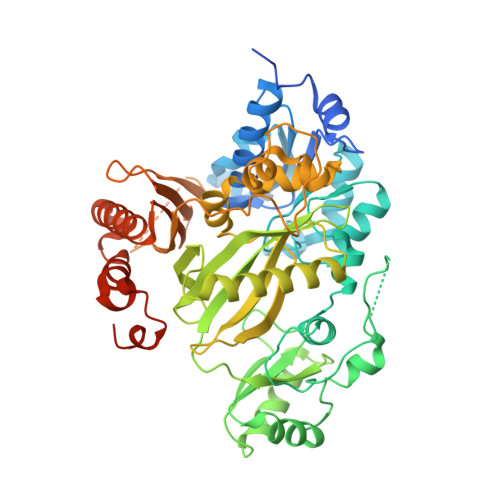
Crystal structure of the biotin carboxylase domain of human acetyl-CoA carboxylase 2.
Cho, Y.S., Lee, J.I., Shin, D., Kim, H.T., Cheon, Y.H., Seo, C.I., Kim, Y.E., Hyun, Y.L., Lee, Y.S., Sugiyama, K., Park, S.Y., Ro, S., Cho, J.M., Lee, T.G., Heo, Y.S.(2008) Proteins 70: 268-272
- PubMed: 17876819
- DOI: https://doi.org/10.1002/prot.21611
- Primary Citation of Related Structures:
2HJW - R&D Center, CrystalGenomics, Inc., Seoul 138-739, Korea.
Organizational Affiliation: