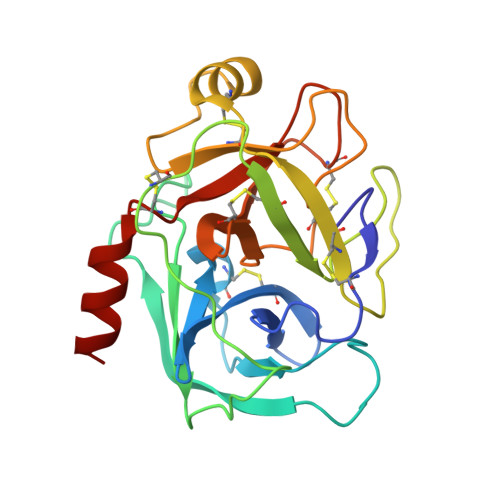
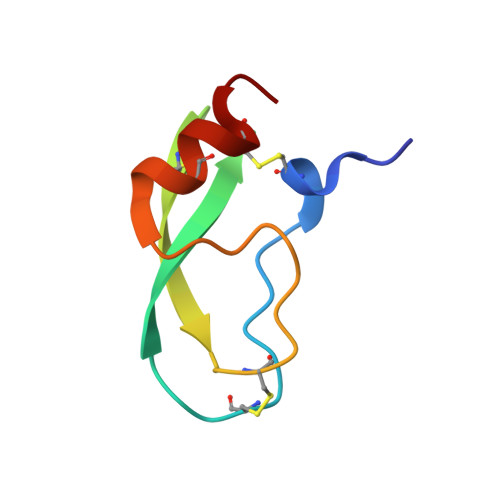
Rigidification of a Flexible Protease Inhibitor Variant upon Binding to Trypsin.
Hanson, W.M., Domek, G.J., Horvath, M.P., Goldenberg, D.P.(2007) J Mol Biology 366: 230-243
- PubMed: 17157870
- DOI: https://doi.org/10.1016/j.jmb.2006.11.003
- Primary Citation of Related Structures:
2FTL, 2FTM - PubMed Abstract:
The Tyr35-->Gly replacement in bovine pancreatic trypsin inhibitor (BPTI) has previously been shown to dramatically enhance the flexibility of the trypsin-binding region of the free inhibitor and to destabilize the interaction with the protease by about 3 kcal/mol. The effects of this replacement on the enzyme-inhibitor interaction were further studied here by X-ray crystallography and isothermal titration calorimetry (ITC). The co-crystal structure of Y35G BPTI bound to trypsin was determined using 1.65 A resolution X-ray diffraction data collected from cryopreserved crystals, and a new structure of the complex with wild-type BPTI under the same conditions was determined using 1.62 A data. These structures reveal that, in contrast to the free protein, Y35G BPTI adopts a conformation nearly identical with that of the wild-type protein, with a water-filled cavity in place of the missing Tyr side-chain. The crystallographic temperature factors for the two complexes indicate that the mutant inhibitor is nearly as rigid as the wild-type protein when bound to trypsin. Calorimetric measurements show that the change in enthalpy upon dissociation of the complex is 2.5 kcal/mol less favorable for the complex containing Y35G BPTI than for the complex with the wild-type inhibitor. Thus, the destabilization of the complex resulting from the Y35G replacement is due to a more favorable change in entropy upon dissociation. The heat capacity changes for dissociation of the mutant and wild-type complexes were very similar, suggesting that the entropic effects probably do not arise from solvation effects, but are more likely due to an increase in protein conformational entropy upon dissociation of the mutant inhibitor. These results define the biophysical role of a highly conserved core residue located outside of a protein-binding interface, demonstrating that Tyr35 has little impact on the trypsin-bound BPTI structure and acts primarily to define the structure of the free protein so as to maximize binding affinity.
- Department of Biology, University of Utah, 257 South 1400 East, Salt Lake City, UT 84112-0840, USA.
Organizational Affiliation: