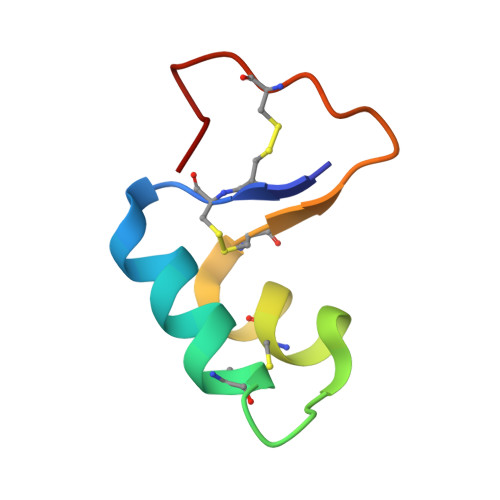
Three-dimensional structure of the water-insoluble protein crambin in dodecylphosphocholine micelles and its minimal solvent-exposed surface.
Ahn, H.C., Juranic, N., Macura, S., Markley, J.L.(2006) J Am Chem Soc 128: 4398-4404
- PubMed: 16569017
- DOI: https://doi.org/10.1021/ja057773d
- Primary Citation of Related Structures:
1YV8, 1YVA, 2EYA, 2EYB, 2EYC, 2EYD - PubMed Abstract:
We chose crambin, a hydrophobic and water-insoluble protein originally isolated from the seeds of the plant Crambe abyssinica, as a model for NMR investigations of membrane-associated proteins. We produced isotopically labeled crambin(P22,L25) (variant of crambin containing Pro22 and Leu25) as a cleavable fusion with staphylococcal nuclease and refolded the protein by an approach that has proved successful for the production of proteins with multiple disulfide bonds. We used NMR spectroscopy to determine the three-dimensional structure of the protein in two membrane-mimetic environments: in a mixed aqueous-organic solvent (75%/25%, acetone/water) and in DPC micelles. With the sample in the mixed solvent, it was possible to determine (>NH...OC<) hydrogen bonds directly by the detection of (h3)J(NC)' couplings. H-bonds determined in this manner were utilized in the refinement of the NMR-derived protein structures. With the protein in DPC (dodecylphosphocholine) micelles, we used manganous ion as an aqueous paramagnetic probe to determine the surface of crambin that is shielded by the detergent. With the exception of the aqueous solvent exposed loop containing residues 20 and 21, the protein surface was protected by DPC. This suggests that the protein may be similarly embedded in physiological membranes. The strategy described here for the expression and structure determination of crambin should be applicable to structural and functional studies of membrane active toxins and small membrane proteins.
- National Magnetic Resonance Facility at Madison, Department of Biochemistry, University of Wisconsin-Madison, 53706-1544, USA.
Organizational Affiliation: