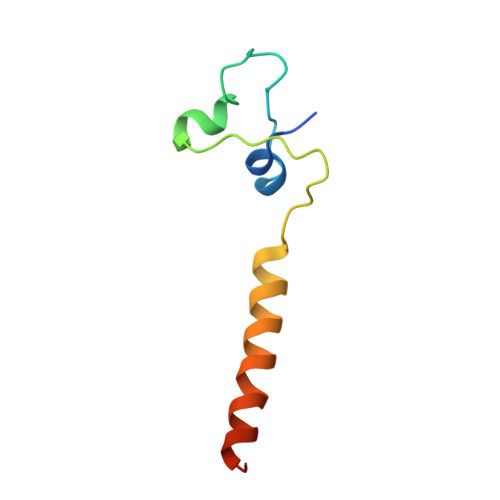
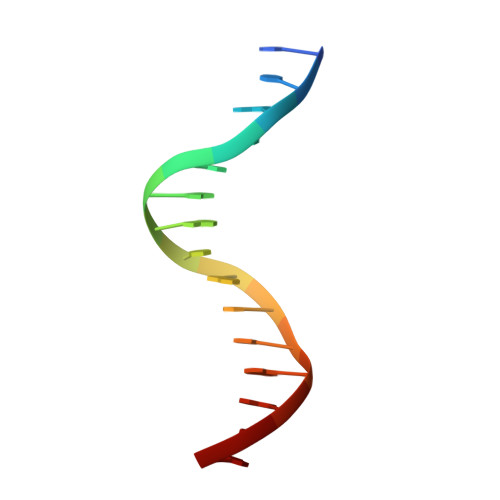
Structure of a Leu3-DNA complex: recognition of everted CGG half-sites by a Zn2Cys6 binuclear cluster protein.
Fitzgerald, M.X., Rojas, J.R., Kim, J.M., Kohlhaw, G.B., Marmorstein, R.(2006) Structure 14: 725-735
- PubMed: 16615914
- DOI: https://doi.org/10.1016/j.str.2005.11.025
- Primary Citation of Related Structures:
2ER8, 2ERE, 2ERG - PubMed Abstract:
Gal4 is the prototypical Zn2Cys6 binuclear cluster transcriptional regulator that binds as a homodimer to DNA containing inverted CGG half-sites. Leu3, a member of this protein family, binds to everted (opposite polarity to inverted) CGG half-sites, and an H50C mutation within the Leu3 Zn2Cys6 binuclear motif abolishes its transcriptional repression function without impairing DNA binding. We report the X-ray crystal structures of DNA complexes with Leu3 and Leu3(H50C) and solution DNA binding studies of selected Leu3 mutant proteins. These studies reveal the molecular details of everted CGG half-site recognition, and suggest a role for the H50C mutation in transcriptional repression. Comparison with the Gal4-DNA complex shows an unexpected conservation in the DNA recognition mode of inverted and everted CGG half-sites, and points to a critical function of a linker region between the Zn2Cys6 binuclear cluster and dimerization regions in DNA binding specificity. Broader implications of these findings are discussed.
- The Wistar Institute, University of Pennsylvania, Philadelphia, Pennsylvania 19104, USA.
Organizational Affiliation: