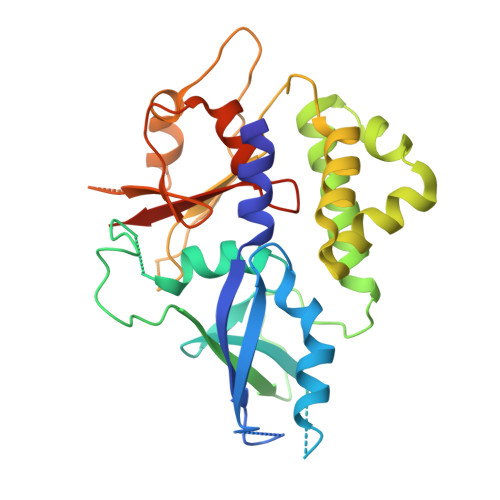
Structure of Atg5.Atg16, a complex essential for autophagy
Matsushita, M., Suzuki, N.N., Obara, K., Fujioka, Y., Ohsumi, Y., Inagaki, F.(2007) J Biological Chem 282: 6763-6772
- PubMed: 17192262
- DOI: https://doi.org/10.1074/jbc.M609876200
- Primary Citation of Related Structures:
2DYM, 2DYO - PubMed Abstract:
Atg5 is covalently modified with a ubiquitin-like modifier, Atg12, and the Atg12-Atg5 conjugate further forms a complex with the multimeric protein Atg16. The Atg12-Atg5.Atg16 multimeric complex plays an essential role in autophagy, the bulk degradation system conserved in all eukaryotes. We have reported here the crystal structure of Atg5 complexed with the N-terminal region of Atg16 at 1.97A resolution. Atg5 comprises two ubiquitin-like domains that flank a helix-rich domain. The N-terminal region of Atg16 has a helical structure and is bound to the groove formed by these three domains. In vitro analysis showed that Arg-35 and Phe-46 of Atg16 are crucial for the interaction. Atg16, with a mutation at these residues, failed to localize to the pre-autophagosomal structure and could not restore autophagy in Atg16-deficient yeast strains. Furthermore, these Atg16 mutants could not restore a severe reduction in the formation of the Atg8-phosphatidylethanolamine conjugate, another essential factor for autophagy, in Atg16-deficient strains under starvation conditions. These results taken together suggest that the direct interaction between Atg5 and Atg16 is crucial to the performance of their roles in autophagy.
- Department of Structural Biology, Graduate School of Pharmaceutical Sciences, Hokkaido University, N-21, W-11, Kita-ku, Sapporo 001-0021, Japan.
Organizational Affiliation: