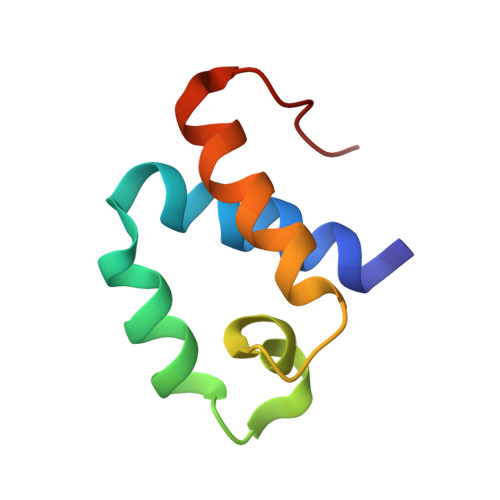
Solution structures of the SURP domains and the subunit-assembly mechanism within the splicing factor SF3a complex in 17S U2 snRNP
Kuwasako, K., He, F., Inoue, M., Tanaka, A., Sugano, S., Guentert, P., Muto, Y., Yokoyama, S.(2006) Structure 14: 1677-1689
- PubMed: 17098193
- DOI: https://doi.org/10.1016/j.str.2006.09.009
- Primary Citation of Related Structures:
2DT6, 2DT7 - PubMed Abstract:
The SF3a complex, consisting of SF3a60, SF3a66, and SF3a120, in 17S U2 snRNP is crucial to spliceosomal assembly. SF3a120 contains two tandem SURP domains (SURP1 and SURP2), and SURP2 is responsible for binding to SF3a60. We found that the SURP2 fragment forms a stable complex with an SF3a60 fragment (residues 71-107) and solved its structure by NMR spectroscopy. SURP2 exhibits a fold of the alpha1-alpha2-3(10)-alpha3 topology, and the SF3a60 fragment forms an amphipathic alpha helix intimately contacting alpha1 of SURP2. We also solved the SURP1 structure, which has the same fold as SURP2. The protein-binding interface of SURP2 is quite similar to the corresponding surface of SURP1, except for two amino acid residues. One of them, Leu169, is characteristic of SF3a120 SURP2 among SURP domains. Mutagenesis showed that this single Leu residue is the critical determinant for complex formation, which reveals the protein recognition mechanism in the subunit assembly.
- Protein Research Group, Yokohama 230-0045, Japan.
Organizational Affiliation: