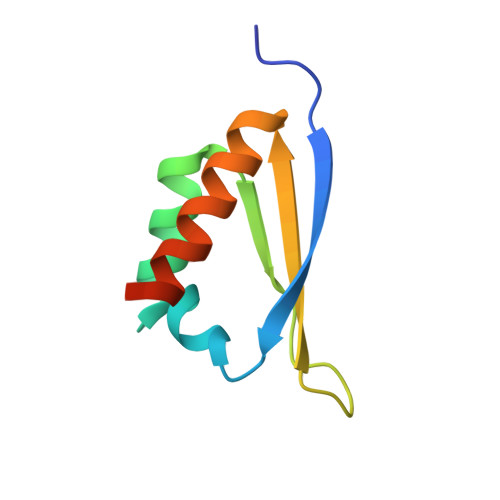
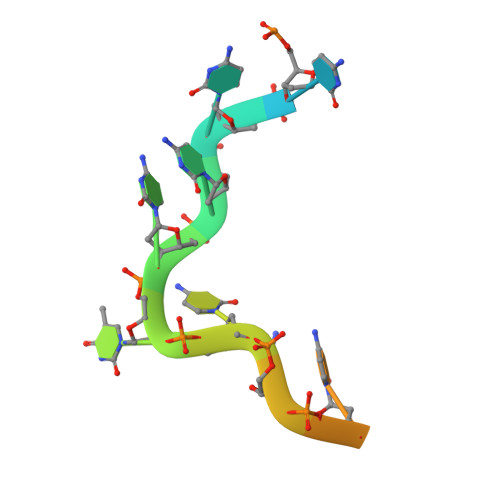
X-Ray Crystallographic and NMR Studies of the Third KH Domain of hnRNP K in Complex with Single-Stranded Nucleic Acids
Backe, P.H., Messias, A.C., Ravelli, R.B., Sattler, M., Cusack, S.(2005) Structure 13: 1055-1067
- PubMed: 16004877
- DOI: https://doi.org/10.1016/j.str.2005.04.008
- Primary Citation of Related Structures:
1ZZI, 1ZZJ, 1ZZK - PubMed Abstract:
The heterogeneous nuclear ribonucleoprotein (hnRNP) K is implicated in multiple functions in the regulation of gene expression and acts as a hub at the intersection of signaling pathways and processes involving nucleic acids. Central to its function is its ability to bind both ssDNA and ssRNA via its KH (hnRNP K homology) domains. We determined crystal structures of hnRNP K KH3 domain complexed with 15-mer and 6-mer (CTC(4)) ssDNAs at 2.4 and 1.8 A resolution, respectively, and show that the KH3 domain binds specifically to both TCCC and CCCC sequences. In parallel, we used NMR to compare the binding affinity and mode of interaction of the KH3 domain with several ssRNA ligands and CTC(4) ssDNA. Based on a structure alignment of the KH3-CTC(4) complex with known structures of other KH domains in complex with ssRNA, we discuss recognition of tetranucleotide sequences by KH domains.
- Grenoble Outstation, European Molecular Biology Laboratory, 6 rue Jules Horowitz, BP 181, F-38042 Grenoble Cedex 9, France.
Organizational Affiliation: