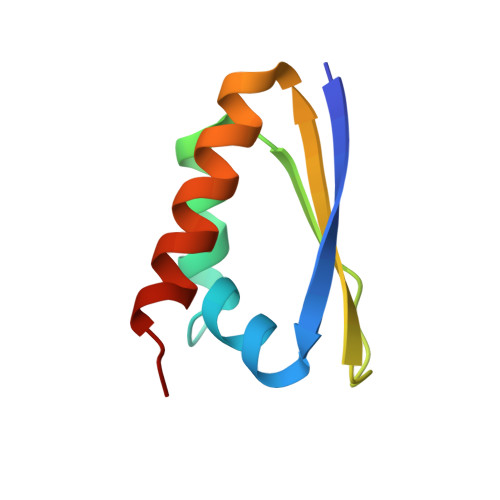
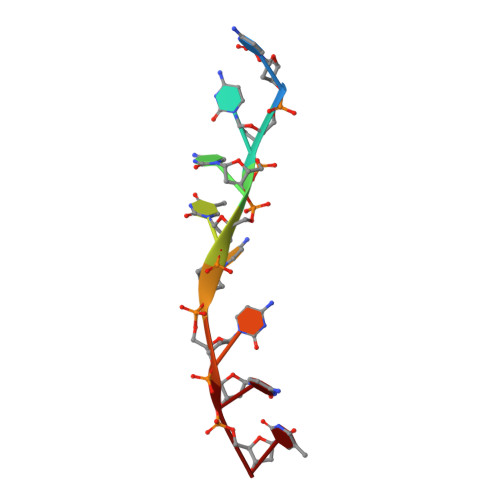
Contribution of the first K-homology domain of poly(C)-binding protein 1 to its affinity and specificity for C-rich oligonucleotides
Yoga, Y.M.K., Traore, D.A.K., Sidiqi, M., Szeto, C., Pendini, N.R., Barker, A., Leedman, P.J., Wilce, J.A., Wilce, M.C.J.(2012) Nucleic Acids Res 40: 5101-5114
- PubMed: 22344691
- DOI: https://doi.org/10.1093/nar/gks058
- Primary Citation of Related Structures:
1ZTG, 3VKE - PubMed Abstract:
Poly-C-binding proteins are triple KH (hnRNP K homology) domain proteins with specificity for single stranded C-rich RNA and DNA. They play diverse roles in the regulation of protein expression at both transcriptional and translational levels. Here, we analyse the contributions of individual αCP1 KH domains to binding C-rich oligonucleotides using biophysical and structural methods. Using surface plasmon resonance (SPR), we demonstrate that KH1 makes the most stable interactions with both RNA and DNA, KH3 binds with intermediate affinity and KH2 only interacts detectibly with DNA. The crystal structure of KH1 bound to a 5'-CCCTCCCT-3' DNA sequence shows a 2:1 protein:DNA stoichiometry and demonstrates a molecular arrangement of KH domains bound to immediately adjacent oligonucleotide target sites. SPR experiments, with a series of poly-C-sequences reveals that cytosine is preferred at all four positions in the oligonucleotide binding cleft and that a C-tetrad binds KH1 with 10 times higher affinity than a C-triplet. The basis for this high affinity interaction is finally detailed with the structure determination of a KH1.W.C54S mutant bound to 5'-ACCCCA-3' DNA sequence. Together, these data establish the lead role of KH1 in oligonucleotide binding by αCP1 and reveal the molecular basis of its specificity for a C-rich tetrad.
- Department of Biochemistry and Molecular Biology, Monash University, Clayton, VIC Australia.
Organizational Affiliation: