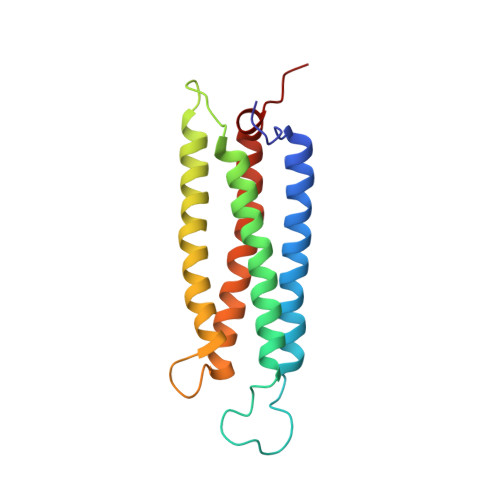
The serine-rich domain from Crk-associated substrate (p130cas) is a four-helix bundle.
Briknarova, K., Nasertorabi, F., Havert, M.L., Eggleston, E., Hoyt, D.W., Li, C., Olson, A.J., Vuori, K., Ely, K.R.(2005) J Biological Chem 280: 21908-21914
- PubMed: 15795225
- DOI: https://doi.org/10.1074/jbc.M501258200
- Primary Citation of Related Structures:
1Z23 - PubMed Abstract:
p130(cas) (Crk-associated substrate) is a docking protein that is involved in assembly of focal adhesions and concomitant cellular signaling. It plays a role in physiological regulation of cell adhesion, migration, survival, and proliferation, as well as in oncogenic transformation. The molecule consists of multiple protein-protein interaction motifs, including a serine-rich region that is positioned between Crk and Src-binding sites. This study reports the first structure of a functional domain of Cas. The solution structure of the serine-rich region has been determined by NMR spectroscopy, demonstrating that this is a stable domain that folds as a four-helix bundle, a protein-interaction motif. The serine-rich region bears strong structural similarity to four-helix bundles found in other adhesion components like focal adhesion kinase, alpha-catenin, or vinculin. Potential sites for phosphorylation and interaction with the 14-3-3 family of cellular regulators are identified in the domain and characterized by site-directed mutagenesis and binding assays. Mapping the degree of amino acid conservation onto the molecular surface reveals a patch of invariant residues near the C terminus of the bundle, which may represent a previously unidentified site for protein interaction.
- Burnham Institute, 10901 N. Torrey Pines Road, La Jolla, CA 92037, USA.
Organizational Affiliation: