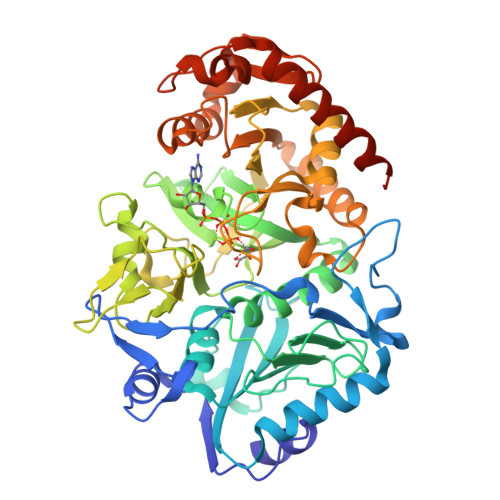
Crystal structure of Anaerobiospirillum succiniciproducens PEP carboxykinase reveals an important active site loop
Cotelesage, J.J.H., Prasad, L., Zeikus, J.G., Laivenieks, M., Delbaere, L.T.J.(2005) Int J Biochem Cell Biol 37: 1829-1837
- PubMed: 15890557
- DOI: https://doi.org/10.1016/j.biocel.2005.03.008
- Primary Citation of Related Structures:
1YTM, 1YVY - PubMed Abstract:
The 2.2 Angstroms resolution crystal structure of the enzyme phosphoenolpyruvate carboxykinase (PCK) from the bacterium Anaerobiospirillum succiniciproducens complexed with ATP, Mg(2+), Mn(2+) and the transition state analogue oxalate has been solved. The 2.4 Angstroms resolution native structure of A. succiniciproducens PCK has also been determined. It has been found that upon binding of substrate, PCK undergoes a conformational change. Two domains of the molecule fold towards each other, with the substrates and metal ions held in a cleft formed between the two domains. This domain movement is believed to accelerate the reaction PCK catalyzes by forcing bulk solvent molecules out of the active site. Although the crystal structure of A. succiniciproducens PCK with bound substrate and metal ions is related to the structures of PCK from Escherichia coli and Trypanosoma cruzi, it is the first crystal structure from this class of enzymes that clearly shows an important surface loop (residues 383-397) from the C-terminal domain, hydrogen bonding with the peptide backbone of the active site residue Arg60. The interaction between the surface loop and the active site backbone, which is a parallel beta-sheet, seems to be a feature unique of A. succiniciproducens PCK. The association between the loop and the active site is the third type of interaction found in PCK that is thought to play a part in the domain closure. This loop also appears to help accelerate catalysis by functioning as a 'lid' that shields water molecules from the active site.
Organizational Affiliation:
Department of Biochemistry, University of Saskatchewan, Saskatoon, Canada.