A simple protocol to estimate differences in protein binding affinity for enantiomers without prior resolution of racemates
Fokkens, J., Klebe, G.(2006) Angew Chem Int Ed Engl 45: 985-989
Experimental Data Snapshot
(2006) Angew Chem Int Ed Engl 45: 985-989
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Thrombin light chain | A [auth L] | 27 | Homo sapiens | Mutation(s): 0 EC: 3.4.21.5 | 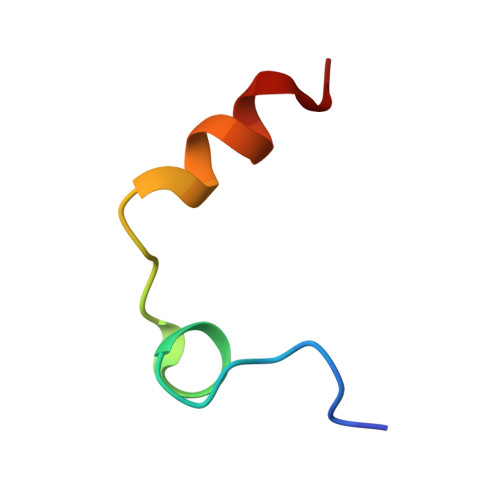 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P00734 (Homo sapiens) Explore P00734 Go to UniProtKB: P00734 | |||||
PHAROS: P00734 GTEx: ENSG00000180210 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00734 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Thrombin heavy chain | B [auth H] | 257 | Homo sapiens | Mutation(s): 0 EC: 3.4.21.5 | 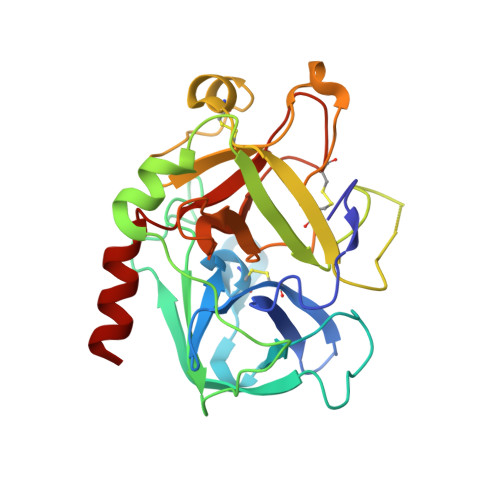 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P00734 (Homo sapiens) Explore P00734 Go to UniProtKB: P00734 | |||||
PHAROS: P00734 GTEx: ENSG00000180210 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00734 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Hirudin | C [auth I] | 10 | Hirudo medicinalis | Mutation(s): 0 | 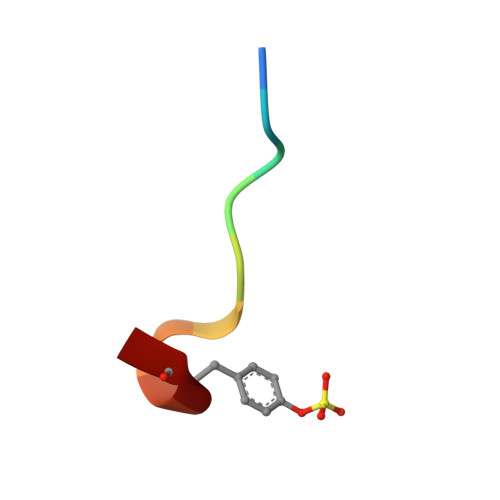 |
UniProt | |||||
Find proteins for P01050 (Hirudo medicinalis) Explore P01050 Go to UniProtKB: P01050 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P01050 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| UIB Query on UIB | D [auth H] | (1R,3AS,4R,8AS,8BR)-4-{5-(PHENYL[1,3]DIOXOL-5-YLMETHYL)-4-ETHYL-2,3,3-TRIMETHYL-6-OXO-OCTAHYDRO-PYRROLO[3,4-C]PYRROL-1-YL}-BENZAMIDINE C26 H32 N4 O3 HQVPEQYGMUJQHM-MSYGRNIXSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| TYS Query on TYS | C [auth I] | L-PEPTIDE LINKING | C9 H11 N O6 S |  | TYR |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 70.521 | α = 90 |
| b = 71.809 | β = 99.87 |
| c = 71.308 | γ = 90 |
| Software Name | Purpose |
|---|---|
| SHELX | model building |
| SHELXL-97 | refinement |
| SCALEPACK | data scaling |
| CNS | phasing |