Structural Dynamics of {alpha}-Actinin-Vinculin Interactions.
Bois, P.R., Borgon, R.A., Vonrhein, C., Izard, T.(2005) Mol Cell Biol 14: 6112-6122
- PubMed: 15988023
- DOI: https://doi.org/10.1128/MCB.25.14.6112-6122.2005
- Primary Citation of Related Structures:
1YDI - PubMed Abstract:
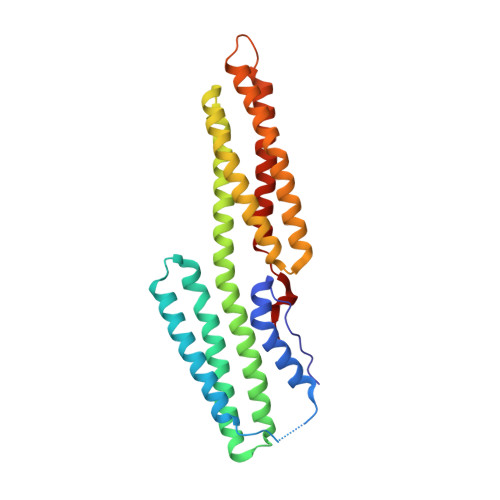
Alpha-actinin and vinculin orchestrate reorganization of the actin cytoskeleton following the formation of adhesion junctions. alpha-Actinin interacts with vinculin through the binding of an alpha-helix (alphaVBS) present within the R4 spectrin repeat of its central rod domain to vinculin's N-terminal seven-helical bundle domain (Vh1). The Vh1:alphaVBS structure suggests that alphaVBS first unravels from its buried location in the triple-helical R4 repeat to allow it to bind to vinculin. alphaVBS binding then induces novel conformational changes in the N-terminal helical bundle of Vh1, which disrupt its intramolecular association with vinculin's tail domain and which differ from the alterations in Vh1 provoked by the binding of talin. Surprisingly, alphaVBS binds to Vh1 in an inverted orientation compared to the binding of talin's VBSs to vinculin. Importantly, the binding of alphaVBS and talin's VBSs to vinculin's Vh1 domain appear to also trigger distinct conformational changes in full-length vinculin, opening up distant regions that are buried in the inactive molecule. The data suggest a model where vinculin's Vh1 domain acts as a molecular switch that undergoes distinct structural changes provoked by talin and alpha-actinin binding in focal adhesions versus adherens junctions, respectively.
- Department of Hematology-Oncology, St. Jude Children's Research Hospital, 332 North Lauderdale Street, Memphis, Tennessee 38105, USA.
Organizational Affiliation: