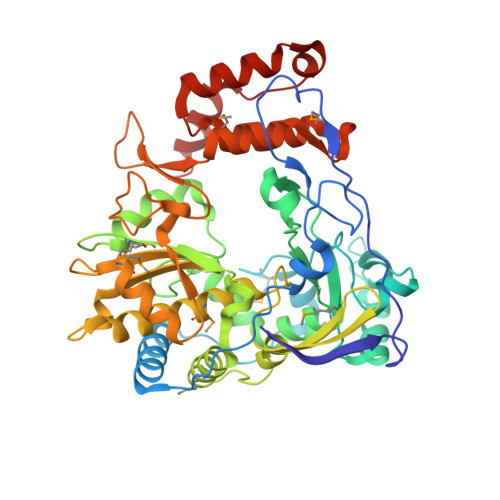
Crystal structure of complete rhinovirus RNA polymerase suggests front loading of protein primer.
Appleby, T.C., Luecke, H., Shim, J.H., Wu, J.Z., Cheney, I.W., Zhong, W., Vogeley, L., Hong, Z., Yao, N.(2005) J Virol 79: 277-288
- PubMed: 15596823
- DOI: https://doi.org/10.1128/JVI.79.1.277-288.2005
- Primary Citation of Related Structures:
1TP7 - PubMed Abstract:
Picornaviruses utilize virally encoded RNA polymerase and a uridylylated protein primer to ensure replication of the entire viral genome. The molecular details of this mechanism are not well understood due to the lack of structural information. We report the crystal structure of human rhinovirus 16 3D RNA-dependent RNA polymerase (HRV16 3Dpol) at a 2.4-A resolution, representing the first complete polymerase structure from the Picornaviridae family. HRV16 3Dpol shares the canonical features of other known polymerase structures and contains an N-terminal region that tethers the fingers and thumb subdomains, forming a completely encircled active site cavity which is accessible through a small tunnel on the backside of the molecule. The small thumb subdomain contributes to the formation of a large cleft on the front face of the polymerase which also leads to the active site. The cleft appears large enough to accommodate a template:primer duplex during RNA elongation or a protein primer during the uridylylation stage of replication initiation. Based on the structural features of HRV16 3Dpo1 and the catalytic mechanism known for all polymerases, a front-loading model for uridylylation is proposed.
- Valeant Pharmaceuticals International, Costa Mesa, California 92626, USA.
Organizational Affiliation: