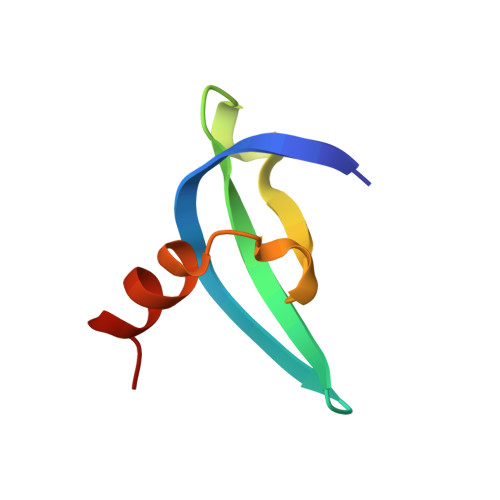
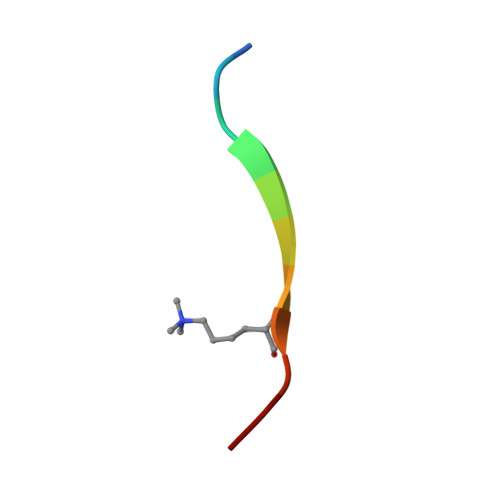
Structural basis for specific binding of Polycomb chromodomain to histone H3 methylated at Lys 27.
Min, J.R., Zhang, Y., Xu, R.-M.(2003) Genes Dev 17: 1823-1828
- PubMed: 12897052
- DOI: https://doi.org/10.1101/gad.269603
- Primary Citation of Related Structures:
1PFB - PubMed Abstract:
The chromodomain of Drosophila Polycomb protein is essential for maintaining the silencing state of homeotic genes during development. Recent studies suggest that Polycomb mediates the assembly of repressive higher-order chromatin structures in conjunction with the methylation of Lys 27 of histone H3 by a Polycomb group repressor complex. A similar mechanism in heterochromatin assembly is mediated by HP1, a chromodomain protein that binds to histone H3 methylated at Lys 9. To understand the molecular mechanism of the methyl-Lys 27 histone code recognition, we have determined a 1.4-A-resolution structure of the chromodomain of Polycomb in complex with a histone H3 peptide trimethylated at Lys 27. The structure reveals a conserved mode of methyl-lysine binding and identifies Polycomb-specific interactions with histone H3. The structure also reveals a dPC dimer in the crystal lattice that is mediated by residues specifically conserved in the Polycomb family of chromodomains. The dimerization of dPC can effectively account for the histone-binding specificity and provides new mechanistic insights into the function of Polycomb. We propose that self-association is functionally important for Polycomb.
- W.M. Keck Structural Biology Laboratory, Cold Spring Harbor Laboratory, Cold Spring Harbor, New York 11724, USA.
Organizational Affiliation: