The Crystal Structure of Dipeptidyl Peptidase IV (CD26) Reveals its Functional Regulation and Enzymatic Mechanism
Engel, M., Hoffmann, T., Wagner, L., Wermann, M., Heiser, U., Kiefersauer, R., Huber, R., Bode, W., Demuth, H.U., Brandstetter, H.(2003) Proc Natl Acad Sci U S A 100: 5063-5068
- PubMed: 12690074
- DOI: https://doi.org/10.1073/pnas.0230620100
- Primary Citation of Related Structures:
1ORV, 1ORW - PubMed Abstract:
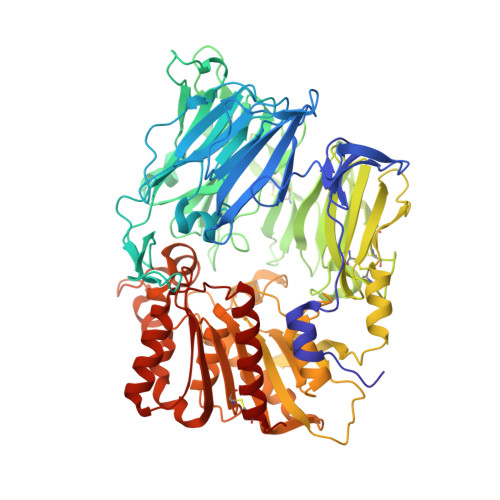
The membrane-bound glycoprotein dipeptidyl peptidase IV (DP IV, CD26) is a unique multifunctional protein, acting as receptor, binding and proteolytic molecule. We have determined the sequence and 1.8 A crystal structure of native DP IV prepared from porcine kidney. The crystal structure reveals a 2-2-2 symmetric tetrameric assembly which depends on the natively glycosylated beta-propeller blade IV. The crystal structure indicates that tetramerization of DP IV is a key mechanism to regulate its interaction with other components. Each subunit comprises two structural domains, the N-terminal eight-bladed beta-propeller with open Velcro topology and the C-terminal alpha/beta-hydrolase domain. Analogy with the structurally related POP and tricorn protease suggests that substrates access the buried active site through the beta-propeller tunnel while products leave the active site through a separate side exit. A dipeptide mimicking inhibitor complexed to the active site discloses key determinants for substrate recognition, including a Glu-Glu motif that distinguishes DP IV as an aminopeptidase and an oxyanion trap that binds and activates the P(2)-carbonyl oxygen necessary for efficient postproline cleavage. We discuss active and nonactive site-directed inhibition strategies of this pharmaceutical target protein.
- Max-Planck-Institut für Biochemie, Abt. Strukturforschung, D-82152 Martinsried, Germany.
Organizational Affiliation: