NMR Solution Structure of the Glucagon Antagonist [desHis1, desPhe6, Glu9]Glucagon Amide in the Presence of Perdeuterated Dodecylphosphocholine Micelles
Ying, J., Ahn, J.-M., Jacobsen, N.E., Brown, M.F., Hruby, V.J.(2003) Biochemistry 42: 2825-2835
- PubMed: 12627948
- DOI: https://doi.org/10.1021/bi026629r
- Primary Citation of Related Structures:
1NAU - PubMed Abstract:
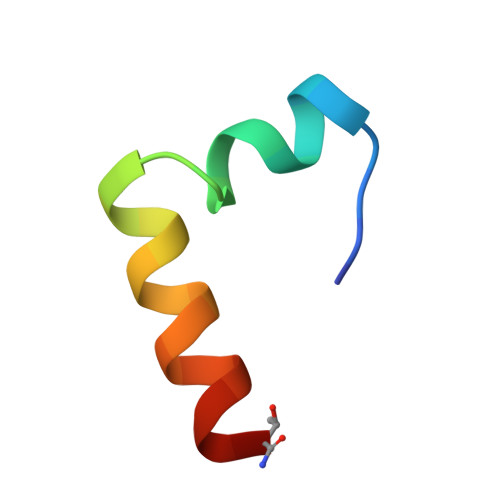
Glucagon, a 29-residue peptide hormone, plays an important role in glucose homeostasis and in diabetes mellitus. Several glucagon antagonists and agonists have been developed, but limited structural information is available to clarify the basis of their biological activity. The solution structure of the potent glucagon antagonist, [desHis1, desPhe6, Glu9]glucagon amide, was determined by homonuclear 2D NMR spectroscopy at pH 6.0 and 37 degrees C in perdeuterated dodecylphosphocholine micelles. The overall backbone root-mean-square deviation (rmsd) for the structured portion (residues 7-29, glucagon numbering) of the micelle-bound 27-residue peptide is 1.36 A for the 15 lowest-energy structures, after restrained molecular dynamics simulation. The structure consists of four regions (segment backbone rmsd in A): an unstructured N-terminal segment between residues 2 and 5 (1.68), an irregular helix between residues 7 and 14 (0.79), a hinge region between residues 15 and 18 (0.54), and a well-defined alpha-helix between residues 19 and 29 (0.33). The two helices form an L-shaped structure with an angle of about 90 degrees between the helix axes. There is an extended hydrophobic cluster, which runs along the inner surface of the L-structure and incorporates the side chains of the hydrophobic residues of each of the amphipathic helices. The outer surface contains the hydrophilic side chains, with two salt bridges (D15-R18 and R17-D21) implied from close approach of the charged groups. This result is the first clear indication of an overall tertiary fold for a glucagon analogue in the micelle-bound state. The relationship of the two helical structural elements may have important implications for the biological activity of the glucagon antagonist.
- Department of Chemistry, University of Arizona, Tucson, Arizona 85721, USA.
Organizational Affiliation: