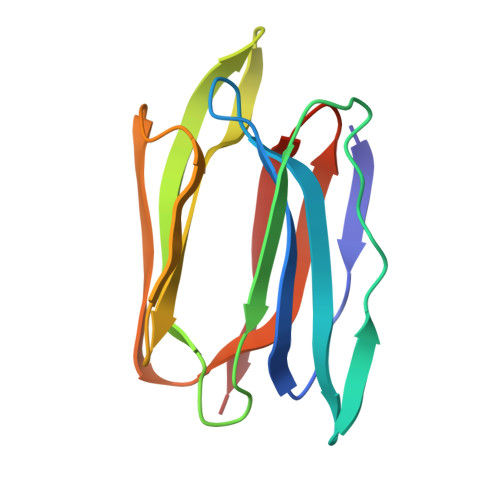
Structural basis for the unusual carbohydrate-binding specificity of jacalin towards galactose and mannose.
Bourne, Y., Astoul, C.H., Zamboni, V., Peumans, W.J., Menu-Bouaouiche, L., Van Damme, E.J., Barre, A., Rouge, P.(2002) Biochem J 364: 173-180
- PubMed: 11988090
- DOI: https://doi.org/10.1042/bj3640173
- Primary Citation of Related Structures:
1KU8, 1KUJ - PubMed Abstract:
Evidence is presented that the specificity of jacalin, the seed lectin from jack fruit (Artocarpus integrifolia), is not directed exclusively against the T-antigen disaccharide Galbeta1,3GalNAc, lactose and galactose, but also against mannose and oligomannosides. Biochemical analyses based on surface-plasmon-resonance measurements, combined with the X-ray-crystallographic determination of the structure of a jacalin-alpha-methyl-mannose complex at 2 A resolution, demonstrated clearly that jacalin is fully capable of binding mannose. Besides mannose, jacalin also interacts readily with glucose, N-acetylneuraminic acid and N-acetylmuramic acid. Structural analyses demonstrated that the relatively large size of the carbohydrate-binding site enables jacalin to accommodate monosaccharides with different hydroxyl conformations and provided unambiguous evidence that the beta-prism structure of jacalin is a sufficiently flexible structural scaffold to confer different carbohydrate-binding specificities to a single lectin.
- AFMB, UMR-CNRS 6098, 31 Chemin Joseph Aiguier, 13402 Marseille Cedex 20, France.
Organizational Affiliation: