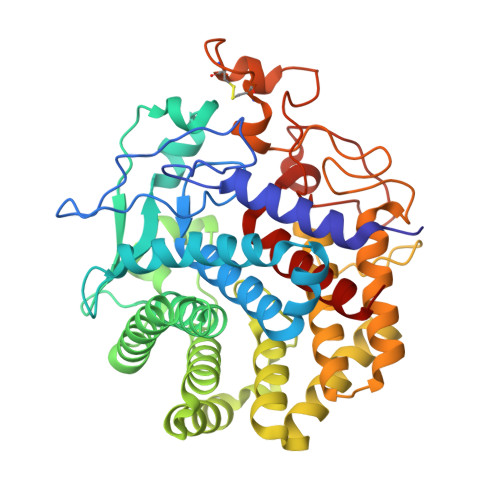
Structure of an endoglucanase from termite, Nasutitermes takasagoensis.
Khademi, S., Guarino, L.A., Watanabe, H., Tokuda, G., Meyer, E.F.(2002) Acta Crystallogr D Biol Crystallogr 58: 653-659
- PubMed: 11914490
- DOI: https://doi.org/10.1107/s0907444902002366
- Primary Citation of Related Structures:
1KS8, 1KSC, 1KSD - PubMed Abstract:
Contrary to conventional wisdom, it has been shown recently that termites do not necessarily depend on symbiotic bacteria to process cellulose. They secrete their own cellulases, mainly endo-beta-1,4-glucanase and beta-1,4-glucosidase. Here, the first structure of an endogenous endoglucanase from the higher termite Nasutitermes takasagoensis (NtEgl) is reported at 1.40 A resolution. NtEgl has the general folding of an (alpha/alpha)(6) barrel, which is a common folding pattern for glycosyl hydrolase family 9. Three-dimensional structural analysis shows that the conserved Glu412 is the catalytic acid/base residue and the conserved Asp54 or Asp57 is the base. The enzyme has a Ca(2+)-binding site near its substrate-binding cleft. Comparison between the structure of the Ca(2+)-free enzyme produced by reducing the pH of the soaked crystal from 5.6 (the pH of optimum enzyme activity) to 2.5 with that of the Ca(2+)-bound enzyme did not show significant differences in the locations of the C(alpha) atoms. The main differences are in the conformation of the residue side chains ligating the Ca(2+) ion. The overall structure of NtEgl at pH 6.5 is similar to that at pH 5.6. The major change observed was in the conformation of the side chain of the catalytic acid/base Glu412, which rotates from a hydrophobic cavity to a relatively hydrophilic environment. This side-chain displacement may decrease the enzyme activity at higher pH.
- Biographics Laboratory, Texas A&M University, Department of Biochemistry and Biophysics, College Station, TX 77843-2128, USA.
Organizational Affiliation: