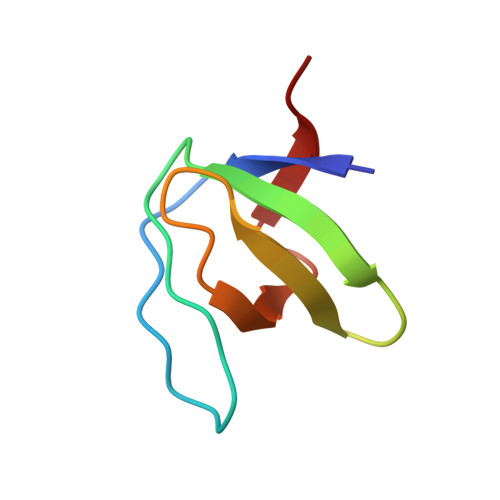
Structure determination of human Lck unique and SH3 domains by nuclear magnetic resonance spectroscopy.
Briese, L., Willbold, D.(2003) BMC Struct Biol 3: 3-3
- PubMed: 12734017
- DOI: https://doi.org/10.1186/1472-6807-3-3
- Primary Citation of Related Structures:
1KIK - PubMed Abstract:
Protein tyrosine kinases are involved in signal transduction pathways that regulate cell growth, differentiation, activation and transformation. Human lymphocyte specific kinase (Lck) is a 56 kDa protein involved in T-cell- and IL2-receptor signaling. Three-dimensional structures are known for SH3, SH2 and kinase domains of Lck as well as for other tyrosine kinases. No structure is known for the unique domain of any Src-type tyrosine kinase. Lck(1-120) comprising unique and SH3 domains was structurally investigated by nuclear magnetic resonance spectroscopy. We found the unique domain, in contrast to the SH3 part, to have basically no defined structural elements. The solution structure of the SH3 part could be determined with very high precision. It does not show significant differences to Lck SH3 in the absence of the unique domain. Minor differences were observed to the X-ray structure of Lck SH3. The unique domain of Lck does not contain any defined structure elements in the absence of ligands and membranes. Presence of the unique domain is not relevant to the three-dimensional structure of the Lck SH3 domain.
- Institut für Molekulare Biotechnologie, 07745 Jena, Germany. briese@imb-jena.de
Organizational Affiliation: