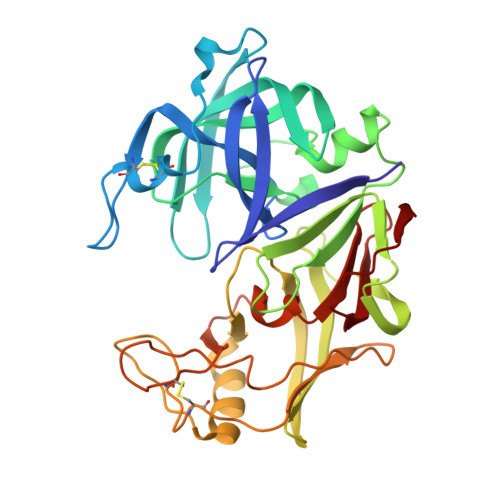
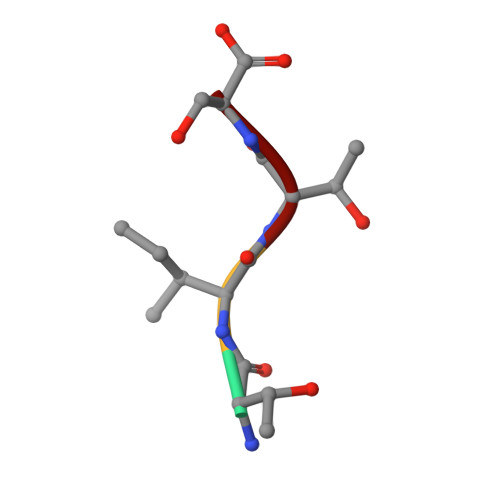
High-resolution structure of the extracellular aspartic proteinase from Candida tropicalis yeast.
Symersky, J., Monod, M., Foundling, S.I.(1997) Biochemistry 36: 12700-12710
- PubMed: 9335526
- DOI: https://doi.org/10.1021/bi970613x
- Primary Citation of Related Structures:
1J71 - PubMed Abstract:
The crystal structure of the secreted aspartic proteinase from Candida tropicalis yeast (SAPT) has been determined to 1.8 A resolution. The classic aspartic proteinase bilobal structure and domain topology is conserved in SAPT, with the substrate binding cleft situated between the two domains. Structural comparisons made with pepsin indicate that insertions and deletions in the primary sequence modify the SAPT structure to create a more spacious substrate binding cleft with altered specificity. An unexpected tetrapeptide has been found to occupy binding sites S1'-S3', and this suggests the order of release of peptide products in the catalytic mechanism of these enzymes. Structural features are considered with regard to previous substrate specificity data.
- Laboratory of Protein Crystallography, Crystallography Research Program, Oklahoma Medical Research Foundation, 825 N.E. 13th Street, Oklahoma City, Oklahoma 73104, USA.
Organizational Affiliation: