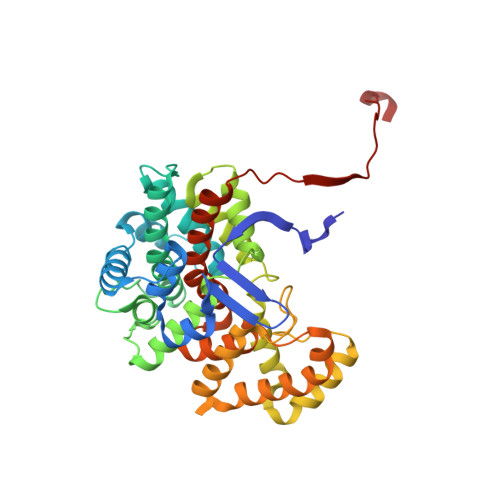
Structural comparison between the open and closed forms of citrate synthase from Thermus thermophilus HB8.
Kanamori, E., Kawaguchi, S., Kuramitsu, S., Kouyama, T., Murakami, M.(2015) Biophys Physicobiol 12: 47-56
- PubMed: 27493854
- DOI: https://doi.org/10.2142/biophysico.12.0_47
- Primary Citation of Related Structures:
1IOM, 1IXE - PubMed Abstract:
The crystal structures of citrate synthase from the thermophilic eubacteria Thermus thermophilus HB8 (TtCS) were determined for an open form at 1.5 Å resolution and for closed form at 2.3 Å resolution, respectively. In the absence of ligands TtCS in the open form was crystalized into a tetragonal form with a single subunit in the asymmetric unit. TtCS was also co-crystallized with citrate and coenzyme-A to form an orthorhombic crystal with two homodimers in the asymmetric unit. Citrate and CoA are found in the active site situated between the large domain and the small domain in all subunit whereas the complex shows two distinct closed conformations, the fully closed form and partially closed form. Structural comparisons are performed to describe conformational changes associated with binding of products of TtCS. Upon binding of citrate, basic residues in the active site move toward citrate and make a hydrogen bond network in the active site, inducing a large-scale rotation of the small domain relative to the large domain. CoA is sandwiched between the small and large domains and then the cysteamine tail is inserted into the active site with a cooperative rotation around mainchain dihedrals in the hinge region connecting helices M and N. According to this rotation these helices are extended to close the active site completely. The considerable flexibility and structural rearrangements in the hinge region are crucial for an ordered bibi reaction in catalysis for microbial CSs.
- Department of Physics, Graduate School of Science, Nagoya University, Nagoya, Aichi 464-8602, Japan.
Organizational Affiliation: