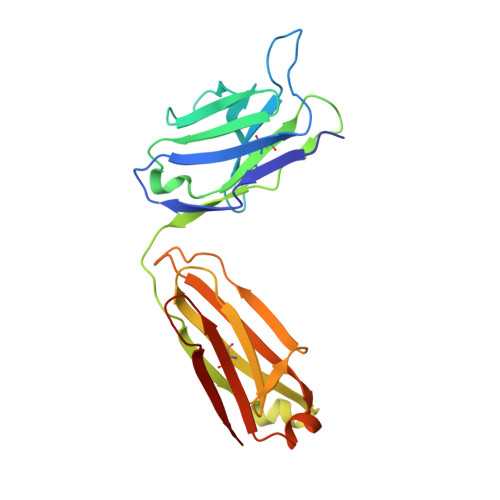
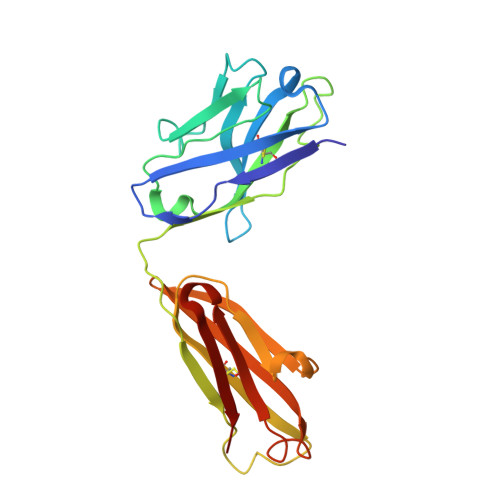
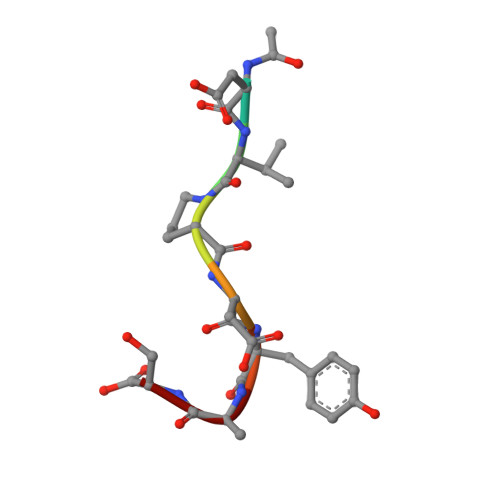
Detailed analysis of the free and bound conformations of an antibody. X-ray structures of Fab 17/9 and three different Fab-peptide complexes.
Schulze-Gahmen, U., Rini, J.M., Wilson, I.A.(1993) J Mol Biology 234: 1098-1118
- PubMed: 8263915
- DOI: https://doi.org/10.1006/jmbi.1993.1663
- Primary Citation of Related Structures:
1IFH - PubMed Abstract:
A new orthorhombic crystal form of Fab 17/9 has been determined in complex with a 7-mer peptide from influenza virus hemagglutinin (HA1 101-107, acetylated and amidated). The three-dimensional structure was resolved to 2.8 A with an improved refinement and better geometry than two previously determined Fab 17/9-peptide (HA1 100-108) complexes, facilitating a detailed description of the Fab-peptide interactions. The binding pockets and the peptide antigen are structurally similar in all three peptide complexes of Fab 17/9. The peptide adopts an extended conformation (residues 100 to 103) and a type I reverse turn (residues 104 to 107). Additionally, the antigenic determinant described here correlates well with previous epitope mapping studies. The structures of the free and antigen bound Fab illustrate the role of induced fit as a mechanism for antibody-antigen recognition. Fab 17/9 undergoes a large conformational change, mainly in the H3 loop, upon peptide binding. As a result, the shape of the binding pocket changes substantially in the liganded Fab. However, the backbone conformations of the other hypervariable loops (L2, L3, H1 and H2) show no significant difference between free and bound structures. The conformation of the L1 loop is also maintained in all structures, but its position relative to the framework varies in different crystal environments. The availability of three X-ray structures of an Fab-peptide complex in three different space groups makes it possible to clearly distinguish between crystal packing and antigen binding as the cause of structural differences. Two distinct H3-loop conformations, free and bound, are observed with no evidence otherwise for multiple conformations of the hypervariable loops (CDRs) or increased flexibility in either the free or bound forms.
- Department of Molecular Biology, Scripps Research Institute, La Jolla, CA 92037.
Organizational Affiliation: