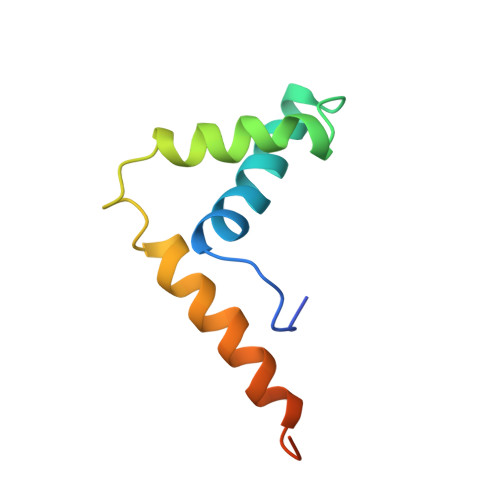
Solution structure and backbone dynamics of the DNA-binding domain of mouse Sox-5.
Cary, P.D., Read, C.M., Davis, B., Driscoll, P.C., Crane-Robinson, C.(2001) Protein Sci 10: 83-98
- PubMed: 11266597
- DOI: https://doi.org/10.1110/ps.32801
- Primary Citation of Related Structures:
1I11 - PubMed Abstract:
The fold of the murine Sox-5 (mSox-5) HMG box in free solution has been determined by multidimensional NMR using (15)N-labeled protein and has been found to adopt the characteristic twisted L-shape made up of two wings: the major wing comprising helix 1 (F10--F25) and helix 2 (N32--A43), the minor wing comprising helix 3 (P51--Y67) in weak antiparallel association with the N-terminal extended segment. (15)N relaxation measurements show considerable mobility (reduced order parameter, S(2)) in the minor wing that increases toward the amino and carboxy termini of the chain. The mobility of residues C-terminal to Q62 is significantly greater than the equivalent residues of non-sequence-specific boxes, and these residues show a weaker association with the extended N-terminal segment than in non-sequence boxes. Comparison with previously determined structures of HMG boxes both in free solution and complexed with DNA shows close similarity in the packing of the hydrophobic cores and the relative disposition of the three helices. Only in hSRY/DNA does the arrangement of aromatic sidechains differ significantly from that of mSox-5, and only in rHMG1 box 1 bound to cisplatinated DNA does helix 1 have no kink. Helix 3 in mSox-5 is terminated by P68, a conserved residue in DNA sequence-specific HMG boxes, which results in the chain turning through approximately 90 degrees.
- Biophysics Laboratories, St. Michael's Building, University of Portsmouth, White Swan Road, Portsmouth PO1 2DT, UK.
Organizational Affiliation: